Open Journal of Veterinary Medicine
Vol.4 No.9(2014), Article
ID:49457,7
pages
DOI:10.4236/ojvm.2014.49021
Analysis of Diagnostic Efficiency of Excretory-Secretory Antigens for Trichinella spiralis and Trichinella nativa in ELISA
Irina Odoevskaya1,2, Sergey Movsesyan2,3, Mikhail Voronin2, Nataya Chiljuta1
1K.I. Skryabin All Russian Institute of Helminthology, Agricultural Academy of Sciences, Moscow, Russian Federation
2Center of Parasitology, A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russian Federation
3Institute of Zoology, Scientific Center of Zoology and Hydroecology, National Academy of Sciences of Armenia, Yerevan, Armenia
Email: odoevskayaim@rambler.ru, movsesyan@list.ru, movsesyan@list.ru
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 June 2014; revised 26 July 2014; accepted 16 August 2014
ABSTRACT
The comparative studies of diagnostic efficiency of excretory-secretory antigens of Trichinella spiralis and Trichinella nativa were performed using blood sera of rats from Wistar line experimentally infected with Arctic trichinellae. For animal infection and antigen preparation Trichinella from muscles of wild carnivorous mammals from Arctic regions of Russia were used. When antigen from T. nativa larvae was used to analyze titers of sera of rats experimentally infected with Arctic Trichinella, a significant increase in efficacy of ELISA was detected. E.g., sera of rats infected with trichinellae from ringed seals retained in ELISA with T. nativa antigen values higher than diagnostic level at titers of 1:6400 - 1:12800, while titer of those same sera when using T. spiralis antigen was no higher than 1:200 - 1:400.
Keywords:ELISA, Trichinella nativa, Diagnostic Efficiency, Laboratory Infection, Wistar Rats, Blood Sera, Excretory-Secretory Antigen

1. Introduction
Epidemiological situation regarding trichinellosis in several regions of Russian Federation (RF) is a stable one, though the problem remains serious. It is especially pronounced in regions of Arctic North where human trichinellosis is clearly connected with that of land and sea mammals. Hunting and fishing are historically the main source of sustenance for natives of the regions, they determine social and economic structure of coastal settlements on Arctic and Pacific ocean shores. An analysis of data on epizootology of trichinellosis at Chukotka territory shows an advisability of broad seroepidemiological studies among natives of the region [1] [2] .
It’s well known that the success of in Vivo immunological diagnostics is mainly determined by quality of an antigen used in enzyme-linked immunosorbent assay (ELISA). Currently at all Russian territories where trichinellosis is endemic, test systems (immune enzyme analysis, non-direct gem agglutination) based on antigens obtained from infective larvae of European strains of T. spiralis circulating throughout biocenosi of North Caucasus, Krasnodar Region, and Belarus Republic are used [3] . However, significant differences in results of seroepidemiological monitoring in infection foci of South and North territories of RF suggest that blood of people infested with “Arctic” trichinellae contains immune complexes which are not revealed by test systems based on Trichinella spiralis antigens [3] [4] . Accordingly, in this study we’ve decided to precede studies of blood sera of Chukotka natives for antibodies specific to T. nativа antigens with comparative analysis of diagnostic efficiency of excretory-secretory antigens of European and Arctic strains of trichinellae in ELISA regarding blood sera from laboratory animals experimentally infected with Arctic isolates of trichinellae.
To work out ELISA parameters we have performed a comparative analysis of efficiency of two immune-enzyme test systems based on T. spiralis and T. nativa antigens, respectively. The sensitivity of these methods is measured through results of the reaction with the same group of blood sera from experimentally infected Wistar line rats. Necessity of such studies is due to the fact that no respondents among the Arctic regions natives whose blood was tested had clinical pattern of trichinellosis, so objective assessment of efficiency of the test using different antigens in ELISA is not possible.
2. Materials and Methods
2.1. Animals
50 rats from Wistar line, weight 160 - 180 g; 40 golden hamsters, weight 100 - 120 g.
• 2.2. Materials
• Trichinella larvae initially obtained from muscles of naturally infected carnivores from Arctic Region and larvae of standard laboratory strain of Trichinella spiralis (from domestic pig of Belarus) passed for many years through laboratory rodents in Skrjabin Institute’s vivarium;
• Excretory-secretory antigens of Trichinella spiralis and Trichinella nativa;
• Blood serum of rats from Wistar line—clinically healthy and experimentally infected with Arctic trichinellae and other helminths (E. granulosus, E. multilocularis, Hymenolepis nana);
• Anti-species conjugate antibodies to rat IgG marked with horseradish peroxidase;
• Necessary reagents and assays for ELISA prepared according to European Union Reference Laboratory for Parasites recommendations.
2.3. Methods
2.3.1. Obtaining Infective Trichinella Larvae
To exclude a possibility of antigenic mimicry affecting an analysis results, larvae for secretion of Arctic Trichinella antigen were obtained from hamsters exclusively, while blood serum for ELISA was taken from rats infected with T. nativa. For simultaneous obtainment of necessary volume of viable infective Trichinella larvae able to produce the antigen all hamsters used were arbitrarily divided into 6 groups of 10 hamsters each. Five groups were infected with Arctic trichinellae (from a blue polar fox (Alopex lagopus), a sled dog (Canis familiaris), Polar bear (Ursus maritimus), wolverine (Gulo gulo) and a ringed seal (Phoca hispida) from Chukotka and Yakut Region)) and one with standard T. spiralis strain (from domestic pig of Belarus). The inequality in distribution of the animals between supra-groups (Arctic Trichinella and T. spiralis) was due to very low reproduction potential of all Arctic Trichinella strains (relative to T. spiralis) shown earlier in 4 passages of specimen of these isolates through laboratory rodents [5] .
Golden hamsters and rats were infected per os with larvae of T. nativa and T. spiralis at dose of 10 larvae per 1 g of live weight. After 1.5 - 2 months the animals were sacrificed through cervical dislocation, skinned, internal organs removed and carcasses digested in artificial gastric liquid. Larvae obtained were rinsed multiple times with sterile saline with added antibiotics according to European Union Reference Laboratory for Parasites recommendations [6] .
Muscle tissues of the experimentally infected animals were studied using method of trichinelloscopy.
2.3.2. Incubation of Infective Larvae
Further work on obtaining the larvae excretory-secretory products was performed in sterile conditions. Trichinella larvae were incubated in Petri dishes in Dulbecco’s Modified Eagle’s Medium (DMEM) substrate with L-glutamine (40 μl/ml) and above-mentioned antibiotics at 38.5˚C in common thermostat with larvae concentration of 5000 per 1 ml of medium.
2.3.3. Obtaining Excretory-Secretory Trichinella Antigens
Protein products were obtained from the medium where larvae were incubated in sterile conditions. The medium with trichinellae protein products was sampled once per 24 hr for 3 - 5 days; after each product sampling initial volume was restored through addition of new medium with L-glutamine. Trichinella larvae viability was daily assessed under microscope, if more than 30% of the larvae were dead, cultivation was stopped. Excretory-secretory products obtained were combined, then dialyzed for 48 hr in distilled water with NaCl added (1:10) at 4˚C, with buffer solution changed each 6 - 8 hrs. After the dialysis the antigen was concentrated using polyethylene glycol (PEG)-8000 at 4˚C for 6 - 8 hrs. The protein concentration was determined using spectrophotometer at wave length of 280 nm after which the antigen was passed through a bacterial membrane with pore diameter of 22 - 24 μm. Thereafter the antigens obtained were used for sensitization of polystyrene plates in ELISA at 5 μg/ml in carbonate buffer saline (pH 9.6) for 16 hours. The antigen solution was kept at −18˚C, or at −70˚C in kelvinator [7] -[9] .
2.3.4. Obtaining and Maintenance of Diagnostic Blood Serum
No less than 1.5 - 2 ml of blood serum were taken from each animal and placed into a thermostat for 1 hour at 37˚C. The resulting blood concentrate was contoured with sterile glass stick and transparent liquid phase sampled. These serum probes were placed into microtubes, marked and kept at +4˚C - +8˚C for no more than 4 days before an analysis. In cases of erythrocytes sediment present, the serum was centrifuged at 3000 rotations/ minute for 10 minutes.
2.3.5. Determining an Optimal Titer of Anti-Species Peroxidase Conjugates
Immunoglobulins from blood serum of intact rats to be used in experiment and clinically healthy donors were obtained through sedimentation using ammonium sulphate (50% - 70% saturation). For titration of anti-species conjugates twofold dilutions in phosphate-saline buffer were prepared, starting with 1:100 and up to 1:25600, ELISA was performed according to standard scheme using rat immunoglobulins adsorbed on polystyrene as an “antigen”. As a result, specific titer of anti-species conjugate against IgG of rats had been found to be 1:6000.
2.3.6. Conditions for Solid-Phase ELISA
The analysis was performed by using indirect variant with polystyrene 96-pits strip microplates from Gosniipolimer, Moscow. For solid phase sensitization excretory-secretory antigens with molecular mass of 29 - 63 kDa were used. Measuring of protein concentration in initial solution was performed using specter-photometer at wave length of 280 nm. Trichinella antigen was placed onto polystyrene strips at 5 μg/ml concentration. A solid phase sensitization continued for 16 - 18 hours at 4˚C, after which pits contents were removed and pits rinsed 3 - 5 times with phosphate-saline buffer. Preparation of non-specific reagents for immune-enzyme reaction: phosphate-saline buffer, buffer solutions for sera and conjugate, 3.3’,5.5’-tetramethylbenzidyne-based substrate solution (TMB), stop-reagent (0.5 M sulphuric acid solution)—were performed by using traditional methods with some modifications.
Results were assessed on spectrophotometer 340/ATC by STL-Labsistems (Austria) with automated reader and vertical light ray at wave length of 450 nm.
2.3.7. Analysis of Sensitivity and Specificity of Excretory-Secretory Antigens from T. nativa and T. spiralis Obtained through ELISA
The activity and specificity of excretory-secretory antigens from T. nativa and T. spiralis were determined through ELISA reaction using blood sera of rats from Wistar line experimentally infected with Trichinella from Arctic isolates obtained from muscles of carnivores from Chukotka and Yakutia, as well as those of rats infected with other helminths (E. granulosus, E. multilocularis, Hymenolepis nana) and clinically healthy rats; samples of the latter two categories were taken from serum bank of Skrjabin’s Institute.
Initially, to work out ELISA parameters we had performed a comparative analysis of efficiency of two immune-enzyme test systems: the first was based on T. spiralis antigen, while the second on that of ‘Arctic’ T. nativa. For this comparative study, ELISA was performed on two plates in parallel, using the same rat sera and reagents but different antigens—T. nativa and T. spiralis.
2.3.8. Detection of Anti-Trichinella Antibodies
Detection of the antibodies was performed in general accordance to methods recommended by European Union Reference Laboratory for Parasites, with some changes and notions shown above [6] .
2.3.9. Statistical Analysis
Extinction value (%) was calculated according to formulae given by Community Reference Laboratory for Parasites [6] .
3. Results and Discussion
A Trichinella larvae count after digestion of carcasses of experimentally infected animals in artificial gastric liquid had shown that parasite load (mean for a group) in hamsters infected with laboratory strain of Т. spiralis was 2317 larvae per 1 g of bone-meat mince. The same parameter was 1.86 larvae/g from ringed seal, 76.12 larvae/g from blue polar fox, 59.64 larvae/g from sled dog, 656.08 larvae/g from Polar bear and 23.75 larvae/g from wolverine. All larvae at muscle stage from carcasses of hamsters infected with Arctic isolates were pooled into one sample for a total of 2.6 ml of viable trichinellae ready for cultivation in artificial medium.
Totally we had obtained about 100 ml of purified excretory-secretory antigen of T. spiralis with protein contents of 580 μg/ml and 23 ml of one from T. nativa, with protein contents of 286 μg/ml. These antigen proteins were later used for sensitization of polystyrene plates and subsequent serological studies of blood sera aimed at finding specific antibodies to T. nativa antigens using ELISA.
Microscopic studies of experimental rats sacrificed after 45 - 60 days p.i. (Figure 1) have shown that rats infected with Arctic Trichinella isolates had most larvae in muscles at various stages of destruction, mainly mummified, the helminths were surrounded with extensive cell infiltrates. There were few living Trichinella larvae in the muscles. Most parasites were at the stage of cell-enzymatic resorption.
As a result of comparative studies of efficiency of immune-enzyme test systems based on T. nativa and T. spiralis antigens significant differences in optical density for the same serum samples when using antigens of different Trichinella species as solid phases have been shown (Figure 2). In such cases mean optical density
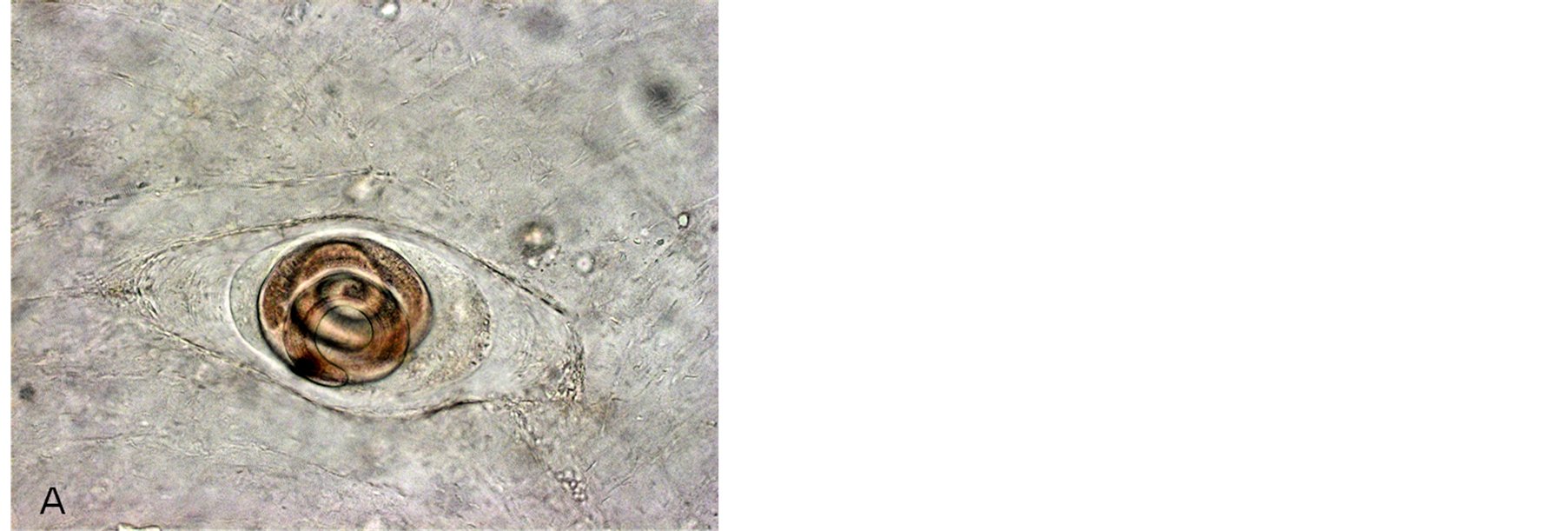
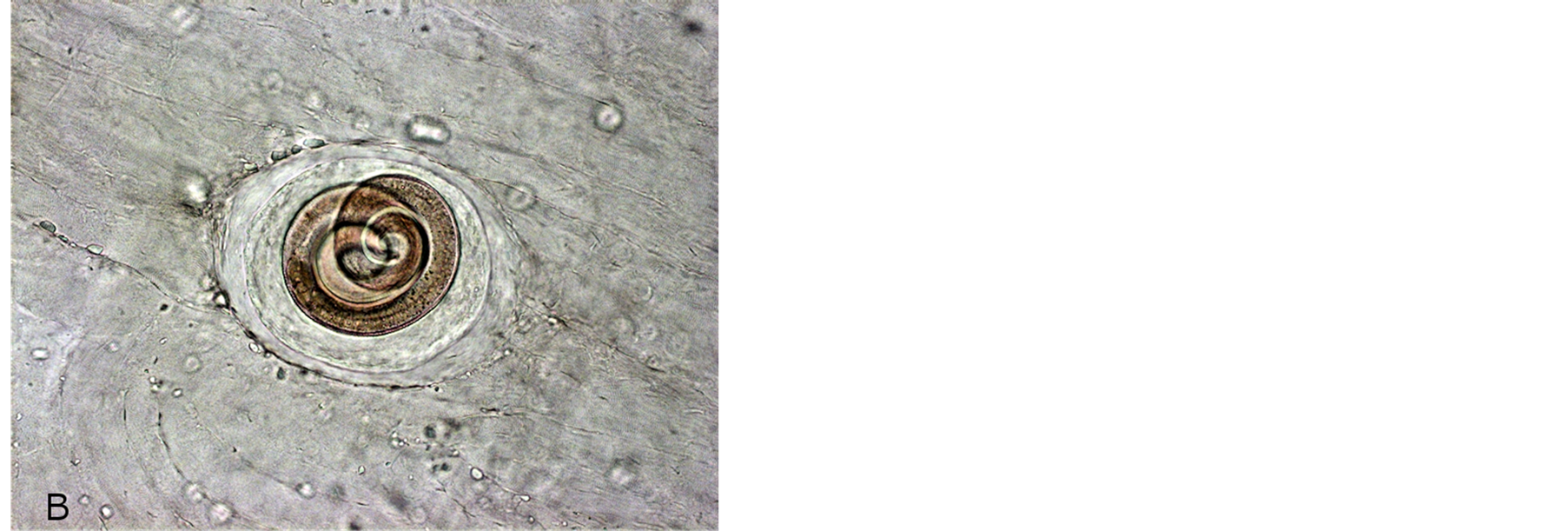
Figure 1. Incapsulated Trichinella larvae in hamster muscles after 3 months p.i. (A) T. spiralis; (B) T. nativa.
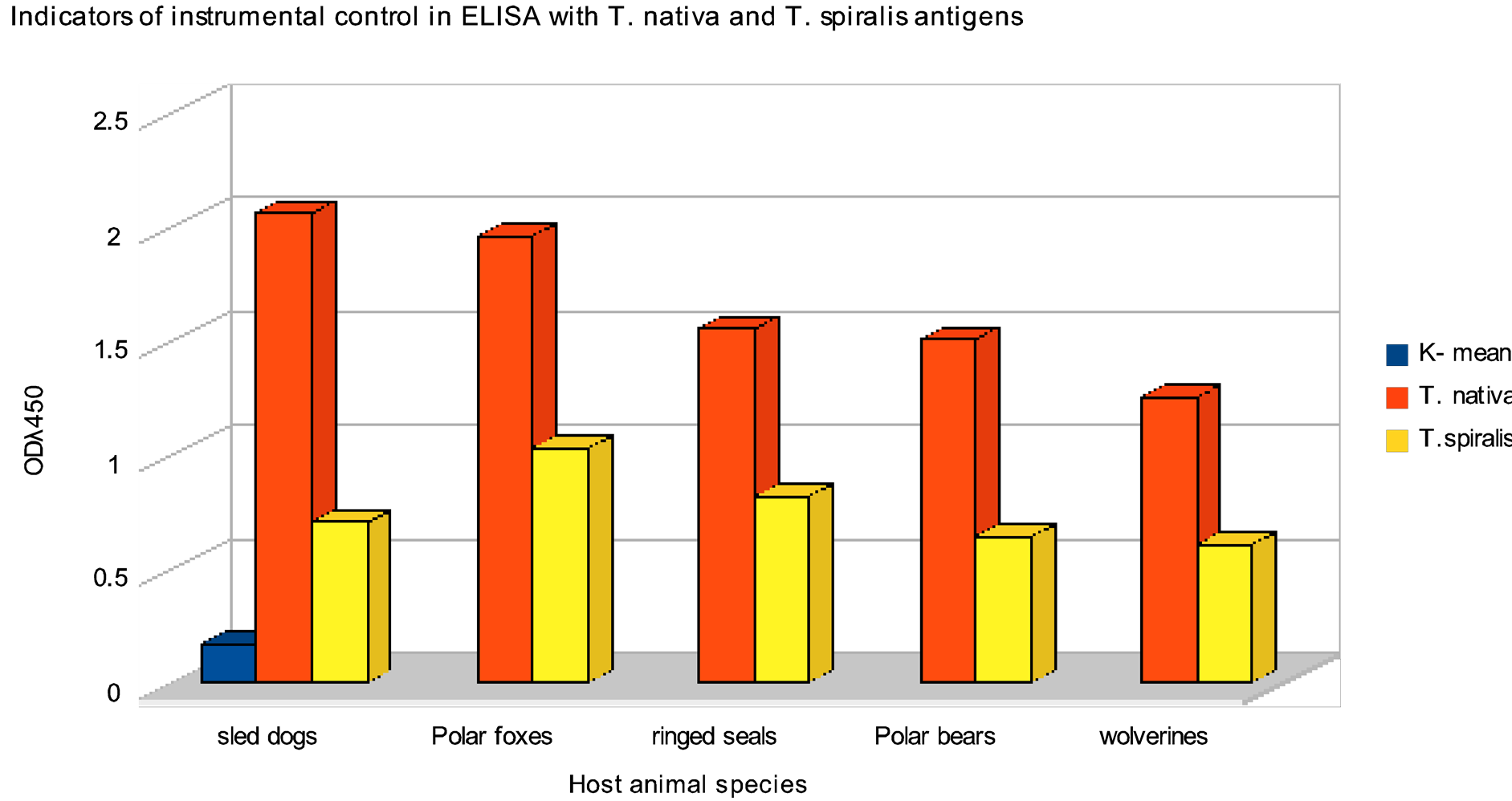
Figure 2. Indicators of instrumental control in ELISA with T. nativa and T. spiralis antigens (OD given is mean for series combined from blood sera of rats experimentally infected with Trichinella obtained from the host species shown on X-axis). K-mean is an indicator of optical density (OD) for negative controls of the reaction.
(OD) of sera in groups of negative controls and rats infected with other helminths were practically the same: 0.127/0.124; 0.120/0.125; 0.099/0.112; 0.175/0.160; 0.303/0.310 (for blood serum of the rat with extensive Echinococcus cyst). All serum probes from rats infected with Arctic trichinellae were considered a “positive control”. They were these probes exactly that have shown significant differences in optical density values when antigens from two Trichinella species were used in ELISA. In all cases ODλ450nm values when using T. spiralis laboratory strain were significantly lower than in cases of T. nativa antigen (see Figure 2). So, sera of rats infected with trichinellae from ringed seals retained in ELISA with T. nativa antigen values higher than diagnostic level at titers of 1:6400 - 1:12800, while titer of those same sera when using T. spiralis antigen was no higher than 1:200 - 1:400. The similar pattern was seen with titers of sera of rats infected with trichinellae from sled dog and polar fox. Of course, most sera of line rats experimentally infected with large dosage of trichinellae (10 larvae/gram) were ELISA-positive at significantly higher than diagnostic level on plates with antigens from both Trichinella species (Table 1).
The results obtained are in good agreement with several earlier works. E.g., Serrano, Perez, Reina, Navarrete [10] had studied a diagnostic efficiency in ELISA of antigens of 5 Spanish Trichinella isolates. The study’s goal was a development of a test system to detect homologous and heterologous infections. Using blood sera from 243 pigs the authors have registered cross reactions between all Trichinella isolates, but homologous sera have shown higher efficiency in ELISA.
Smith and Snowdon [11] have studied diagnostic efficiency of ELISA using blood sera of pigs vaccinated and infected with T. nativa and T. spiralis. In all cases humoral cross reaction had been detected. However, in all cases of homologous sera being used, diagnostic levels of OD five or more times higher than negative control level had been shown, while heterologous blood sera had shown OD level high, but not reaching this diagnostic level.
Sukura et al. [12] have shown using Western blot with T. spiralis and T. nativa antigens obvious cross reactivity with sera of infected raccoon dogs. However, antigens with molecular mass of 40 - 76 kDa reacted with homologous sera much stronger. It should be noted that this experiment have shown impossibility of early detection of Trichinella infection using heterologous antigens. For example, usage of blood sera with homologous antigens allowed detecting specific antibodies two weeks earlier than with heterologous antigens in Western blot and one week earlier in ELISA. At later stage (12 weeks p.i.) indexes of OD in ELISA were significantly higher when using homologous components than with heterologous ones.
Table 1. Diagnostic efficiency of ELISA with sera of Wistar rats experimentally infected with Arctic Trichinella.
4. Conclusion
So, both experimental and literature data clearly demonstrate that in large-scale seroepidemiological studies at conditions far from laboratory standard, such a difference between sensitivity when using heterologous and homologous antigens may lead to a significant decrease in the test efficiency, with many positive sera samples falling behind the method’s sensitivity threshold or becoming “doubtful”. So, it is necessary when performing epidemiological monitoring in trichinellosis foci in Arctic to use an excretory-secretory antigen from larvae of Arctic natural isolates of T. nativa as an active component for ELISA.
Acknowledgements
This study was supported by the grant 14-16-00026 from Russian Scientific Foundation.
The authors would like to thank the journal’s reviewers for their kind critical comments and opportunity to improve the English text.
References
- Bukina, L.A. (2011) Ecologicheskie zakonomernosti tsirkulyatsii trichinell na morskikh poberezhyakh Chukotki. Medical Parasitology, 4, 39-42.
- Odoevskaya, I.M., Movsesyan, S.O. and Panayotova-Pencheva, M. (2013) Comparative Evaluation of the Immunoenzyme Reaction Effect in Application of Trichinella spiralis and Trichinella nativa Antigens. Comptes rendus de l’Academie bulgare des Sciences, 66, 439-444.
- Starkova, T.V. and Poletaeva, O.G. (1996) Otsenki razlichykh metodov instrumentalnogo utchyota resultatov IFA s antigenom trichinell. Proceedings of 7th Scientific Conference on Human and Animal Trichinellosis, Moscow, 2-3 October 1996, 93-95.
- Popov, M.A., Vaserin, Yu.I., Nagorny, S.A. and Tverdokhlebova, T.I. (2002) Diagnostika trichinellosa na Severnom Caucase. Theory and Practice of Fighting Parasitic Diseases, Moscow, 22-23 May 2002, 246-247.
- Odoevskaya, I., Kurnosova, O., Rudenskaya, Yu., Filipova, I., Movsesyan, S. and Bankov, I. (2010) Peculiarities of Parasite-Host Relations in Experimental Infection of Laboratory Rodents wits Arctic Strains of Trichinella nativa. Comptes rendus de l’Academie bulgare des Sciences, 63, 723-732.
- European Union Reference Laboratory for Parasites (2006) Test Method Is Detection of Anti-Trichinella Antibodies in Swine Serum by Indirect ELISA.
http://www.iss.it/crlp/?lang=2&id=54&tipo=33 - Gamble, H.R., Pozio, E., Bruschi, F., Nockler, K., Kapel, C.M.O. and Gajadhar, A.A. (2004) International Commission on Trichinellosis: Recommendations on the Use of Serological Tests for the Detection of Trichinella Infection in Animals and Man. Parasite, 11, 3-13.
- Gomez-Morales, M.A., Ludovisi, A., Amati, M., Cherchi, S., Pezotti, P. and Pozio, E. (2008) Validation of an Enzyme-Linked Immunosorbent Assay for the Diagnosis of Human Trichinellosis. Clinical and Vaccine Immunology, 15, 1723-1729. http://dx.doi.org/10.1128/CVI.00257-08
- Odoevskaya, I.M., Kurnosova, O.P., Movsessyan, S.O. and Bankov, I. (2007) Immunochemical Analysis of Excretory-Secretory Proteins and Antigens of Trichinella spiralis Larvae. Experimental pathology and parasitology, 10, 43-50.
- Serrano, F., Perez, E., Reina, D. and Navarrete, I. (1992) Trichinella Strain, Pig Race and Other Parasitic Infections as Factors in the Reliability of ELISA for the Detection of Swine Trichinellosis. Parasitology, 105, 111-115. http://dx.doi.org/10.1017/S0031182000073753
- Smith, H.J. and Snowdon, K.E. (1987) Detection of Trichinella spiralis nativa Antibodies in Porcine Sera by ELISA Using T. spiralis spiralis Excretory-Secretory Antigen. Canadian Journal of Veterinary Research, 51, 413-414.
- Sukura, A., Nareaho, A., Mikkonen, T., Niemi, M. and Oivanen, L. (2002) Trichinella nativa and T. spiralis Induce Distinguishable Histopathologic and Humoral Responses in the Raccoon Dog (Nyctereutes procyonoides). Veterinary Pathology, 39, 257-265. http://dx.doi.org/10.1354/vp.39-2-257


