Journal of Sustainable Bioenergy Systems
Vol.3 No.1(2013), Article ID:28995,6 pages DOI:10.4236/jsbs.2013.31001
Optimization of Biodiesel Production from Waste Vegetable Oil Assisted by Co-Solvent and Microwave Using a Two-Step Process
1Department of Business Administration, Kun-Shan University, Tainan, Taiwan
2Department of Environment Engineering, Kun-Shan University, Tainan, Taiwan
Email: cclin@mail.ksu.edu.tw, *johnson@mail.ksu.edu.tw
Received January 22, 2013; revised February 25, 2013; accepted March 15, 2013
Keywords: Waste Vegetable Oil; Co-Solvent; Microwave; Two-Step Process
ABSTRACT
The two-step catalyzing process for biodiesel production from waste vegetable oil was assisted by both co-solvent and microwave irradiation. Central composite design (CCD) was employed to optimize the reaction conditions. Optimal reaction conditions of the first step were alcohol to oil molar ratio of 9:1, catalyst (H2SO4) amount 1 wt%, reaction temperature 333 K, and reaction time 7.5 minutes; while for the second step, optimal reaction conditions were alcohol to oil molar ratio 12:1, catalyst (NaOH) amount 1 wt%, reaction temperature 333 K, and reaction time 2.0 minutes. The total reaction time was 9.5 min and the conversion rate of fatty acid methyl esters (FAMEs) achieved was 97.4%. The total reaction time was shorter than previous studies. Therefore, the co-solvent and microwave assisted two-step catalyzing process has a potential application in producing biodiesel from waste vegetable oil.
1. Introduction
Vegetable oil has been considered for a long time as an alternative energy source to produce biodiesel. The conversion of vegetable oils into biodiesel is a potential solution to the increasing demand for energy and environmental concerns [1]. But, using refine vegetable oil to produce biodiesel will reduce the edible oil. Thus, the use of waste vegetable oil instead of refined vegetable oil to produce biodiesel is an effective way to reduce the raw material cost and reduce food shortages. Additionally, using waste vegetable oil could also help solve the problem of waste oil disposal [2,3]. So far, only a minor proportion of the waste vegetable oil has been collected, hence the waste vegetable oil is still causing ecological and economic problems. Therefore, if the waste vegetable oil is used to produce biodiesel, waste vegetable oil treatment may be reduced and the pollution problems caused by this waste vegetable oil may be solved.
Waste vegetable oil usually contains more than 0.5% of free fatty acids (FFAs) which make saponification reaction occur when combined with alkali catalysts and therefore reduces the biodiesel production rate. In order to solve this problem, acid catalysts can be used. However, transesterification reaction using acidic catalysts requires a longer reaction time.
In manufacture of biodiesel, two kinds of catalysts which can be used include acidic and alkaline catalysts. Alkaline catalysts, such as sodium hydroxide, potassium hydroxide or alkaline oxides, usually take place faster than the acidic catalysts do, and they are also more widely used in business [4]. However, the nature of waste vegetable oil is so different from that of the refined vegetable oil, usually in the process of boiling at high temperatures such as viscosity, density and saponification value. Hydrolysis of triglycerides rapidly increases the content of FFAs more in waste vegetable oil than in the refined vegetable oil [5]. Alkaline catalysts are usually chosen to shorten the reaction time and gain high conversion rate when the FFA content is less than 0.5% and vice versa, when the FFA content is higher than 0.5%, acidic catalysts are better selection since they can overcome the saponification problem which is made by the alkaline catalysts [6,7].
In the treatment process of oils which have high content of FFAs (such as waste vegetable oil, animal fats), acidic catalysts show a very good role [8]. However, acid catalysis is about 4000 times slower than alkali catalysis, so it will take more time when using the acid catalyzed reaction.
A two-step catalytic reaction was introduced by Ghadge et al. [9], in which biodiesel was produced from an oil containing 19% of FFAs. The biodiesel conversion rate reach to 98% which was high enough to meet the specified value of the European Union standards.
Microwave has been used as an effective method to accelerate the transesterification process to produce biodiesel by adding heat to the reaction. When the reaction is under the effect of microwave, high yield can be obtained faster and the reaction time is significantly shortened [10,11].
In order to improve the transesterification efficiency, it is very important to find a way to mix well the liquid reactants, especially oil and alcohol, which have very much different polarity and density. Co-solvents, for example simple ethers, such as tetrahydrofuran (THF), diethyl ether, diisopropyl ether, methyl tertiary butyl ether, could be used as a remedy to make the reaction system homogeneous. In this way, they could effectively improve the response rate as they could reduce the surface tension between alcohol and oil [12].
The main aim of this study was to obtain the optimal reaction conditions (alcohol/oil molar ratio; amount of catalyst used; and reaction time) for biodiesel production from waste vegetable oil by a two-step catalyzing process assisted by co-solvent (THF) and microwave irradiation.
2. Methods
2.1. Materials
Sulfuric acid (H2SO4) 99% and alcohol 99.8% were purchased from Shimakyu Co. Ltd. (Japan). Sodium hydroxide (NaOH) 98% was purchased from Mallinckrodt Co. Ltd. (USA). Methyl laurate and acetic acid were purchased from Fluka Co. Ltd. (USA). The waste vegetable oil samples were provided from local restaurants. Residual food in the waste vegetable oil used in this study was removed by filtering.
2.2. Equipment
The used microwave equipment was Milestone Ethos 900, which could offer microwave power from 0 - 900 W. The used chromatography equipment to analyze biodiesel samples was the Shimadzu GC-MS Model QP- 2010 and DB-225 ms capillary column (0.25 mm × 30 m) J&W Scientific. Every 0.5 gram sample consisted of an internal standard of 0.05 gram methyl laurate and 10 ml of hexane, which were well mixed together. Then only 1 μl of the sample was injected into the GC system. GC conditions were: injection port temperature 553 K, nitrogen flow rate of 45 ml/min, air flow rate of 450 ml/min, split ratio 1:20, detector temperature 573 K. The system was programmed to maintain 483 K for 4 minutes, and then increase the temperature at the rate of 4 K/min until 513 K before maintaining this high temperature within 8 minutes.
At the end of this step, biodiesel floated up and was collected and washed with deionized water and finally tested by gas chromatography (GC) analysis for the conversion rate.
2.3. Experimental Procedure
A co-solvent (THF) and microwave assisted two-step catalyzing process was adopted for producing the biodiesel from waste vegetable oil. At first, sulfuric acid was used as a catalyst for the esterification process, and then hydroxide was used to catalyze the transesterification process. In addition, reaction temperature was only 2 K above the boiling point of alcohol, thus alcohol could be collected completely to be reused by distillation at the end of the reaction.
In the first step, the waste vegetable oil was mixed with alcohol and the alcohol to oil ratio set at 3:1 - 15:1 (3:1, 6:1, 9:1, 12:1, and 15:1), with additional catalyst which was at 0.5 - 1.5 wt% (0.5, 0.75, 1.0, 1.25, and 1.5). The whole mixture was then added by a co-solvent to form a homogeneous phase before being put into the Teflon bottle of a microwave system. The microwave system supported heat to the reaction at the power set at 300 W to maintain the reaction temperature at 323 - 343 K during the whole reaction time (12.5 minutes). When the reaction ended, after being washed several times with deionized water, the collected product was then used for the second step.
In the second step, the reaction conditions were alcohol to oil ratio 12:1, catalyst amount 1%, reaction temperature 333 K and reaction time from 0.5 to 2.5 minutes.
2.4. Analytical Methods
In chemistry, acid value is the mass of potassium hydroxide (KOH) in milligrams that is required to neutralize one gram of chemical substance. The acid value (KOH mg/g) and aponification value (KOH mg/g) were determined by a standard titrimetry method (AOCS: American Oil Chemists’ Society). The molecular weight of the waste vegetable oil was calculated from its acid value and aponification value. The experimental results in terms of the acid value, aponification value and molecular weight were 4.36, 287.36 and 588.67, respectively.
The conversion rate of crude biodiesel was calculated according to the area of FAME as expressed in the following equation [13]:
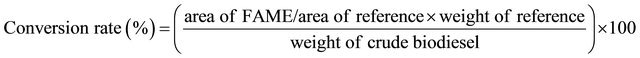 (1)
(1)
3. Results and Discussion
3.1. First Step
In the first step, effects of co-solvent (THF), reaction temperature, value of alcohol to oil ratio and amount of catalyst on the performance of the process were considered. The main purpose of the first step was to reduce the acid value lower than 2.0 KOH mg/g.
3.1.1. Effect of Co-Solvent (THF)
Under the same experimental conditions, which were: alcohol to oil molar ratio of 9:1, amount of sulfuric acid (H2SO4) 1 wt%, reaction temperature 333 K. Figure 1 and Figure 2 showed the effects of the THF on the acid value and the conversion rate. It can be seen that when THF was not added, the acid value (Figure 1) was maintained at high level (about at 4.2 KOH mg/g) and the conversion rate (Figure 2) was maintained at 0% within the whole reaction time (12.5 minutes). Meanwhile, with the THF was added, the acid value was reduced very fast and the conversion rate of biodiesel increased gradually with time although at very low value (<4%).
This was because acidic catalyst was used, with which required reaction time should be much longer than 30 minutes [14]. This also revealed that much more time and energy would be spent if one followed this reaction condition setup. For this reason, a two-step reaction should be used to shorten the reaction time and to effectively improve the conversion rate [15].
Figure 1 also shows the acid value was lower than 2.0 when reaction time at 7.5 minutes. Therefore, the optimal
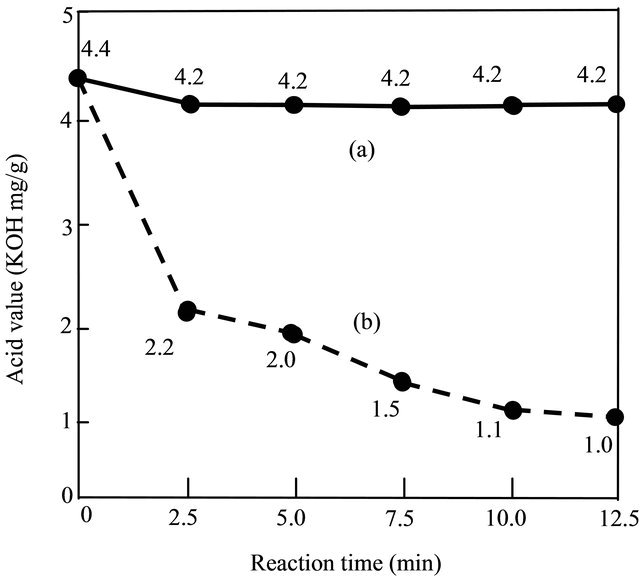
Figure 1. Effect of the THF on the acid value in the first step. (a) Without added THF; (b) Added THF.
reaction in the first step should be 7.5 minutes.
3.1.2. Effect of Alcohol/Oil Molar Ratio
We increased the alcohol/oil molar ratio from 3:1 to 15:1. As seen in Table 1, the acid value was started lower than 2.0 KOH mg/g at alcohol/oil molar ratio 9:1. Therefore, the optimal alcohol/oil molar ratio was 9:1.
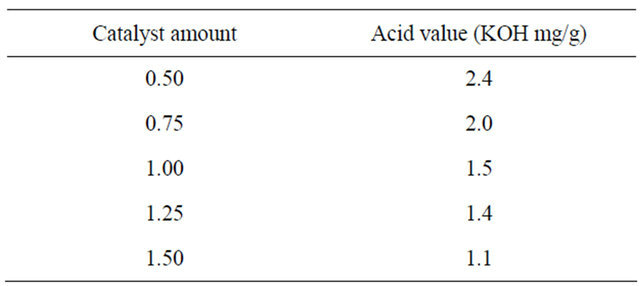
3.1.3. Effect of Catalyst (H2SO4) Amount
We increased the catalyst amount from 0.5 to 1.5. As seen in Table 2, the acid value was started lower than 2.0 at catalyst amount 1.0. Therefore, the optimal catalyst amount was 1.0.
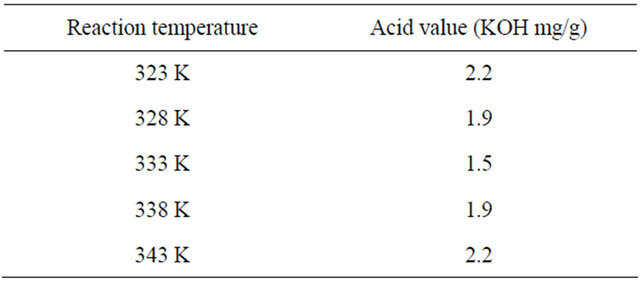
3.1.4. Effect of Reaction Temperature
The effect of the reaction temperature on the acid value is shown in Table 3. When the temperature increased
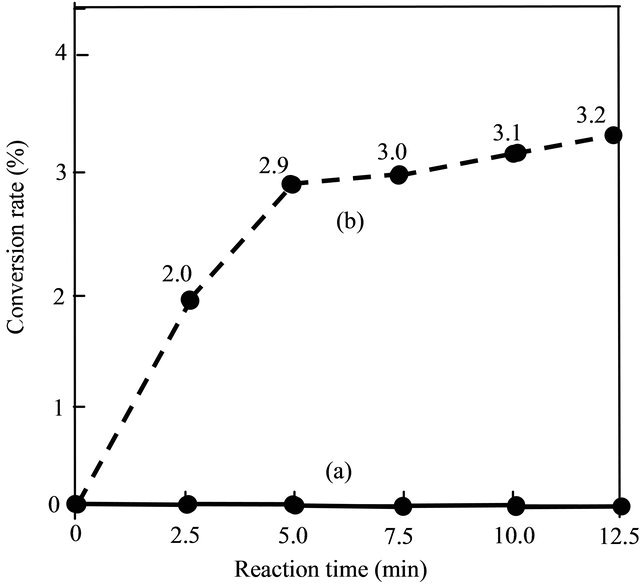
Figure 2. Effect of the THF on the conversion rate in the first step. (a) Without added THF; (b) Added THF.
Table 1. Effects of alcohol/oil molar ratio on the acid value in the first step.
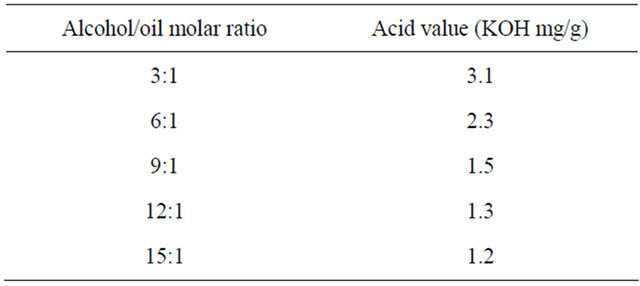
Note: Reaction conditions: sulfuric acid amount 1 wt%, reaction time 7.5 min, reaction temperature 333 K. The original acid value of waste vegetable oil is 4.4 KOH mg/g.
Table 2. Effects of catalyst amount on the acid value in the first step.
Note: Reaction conditions: alcohol/oil molar ratio 9:1, reaction time 7.5 min, reaction temperature 333 K. The original acid value of waste vegetable oil is 4.4 KOH mg/g.
Table 3. Effects of reaction temperature on the acid value in the first step.
Note: Reaction conditions: alcohol/oil molar ratio 9:1, sulfuric acid amount 1.0 wt%, reaction time 7.5 min. The initial acid value of the waste vegetable oil is 4.4 KOH mg/g.
from 323 to 333 K, the solubility of alcohol in the oil increased, the homogeneous degree of the reaction system increased allowing free fatty acid to mix better with methanol and the mass transfer resistance of the twophase reactants reduced. However, when the temperature exceeded 333 K, the acid value was increased. This was mainly due to the boiling temperature 337.5 K of alcohol. When the reaction temperature was near this point, e.g. 338 K, alcohol started evaporating from the reactant mixture resulting in high acid value. The acid value reduced even more when the reaction temperature increased to 343 K because at this time the temperature was higher than the boiling point of both THF and alcohol, thus both THF and alcohol quickly evaporated from the mixture leading to not only reduction of reactant (alcohol) but also reduction mixing level between the two phases.
As a result, the homogeneous phase system of reactants became a two-phase system. Therefore, the most suitable reaction temperature must be 333 K.
3.2. Second Step
In the second step, central composite design (CCD) was used, which is generally the best design for optimization [16]. The uncoded levels of independent variables and experimental results are summarized in Table 4.
Table 4. CCD uncoded levels of independent variables and experimental results.
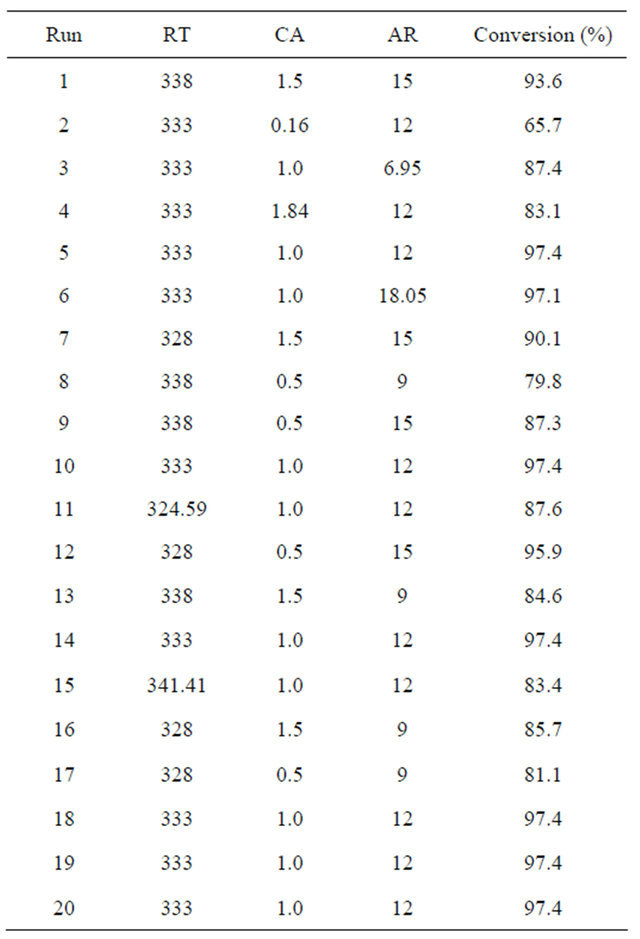
The three variables selected were reaction temperature (RT), catalyst amount (CA), and alcohol/oil molar ratio (AR), and their respective levels were as follows: reaction temperature (324.59 K - 341.41 K), catalyst (NaOH) amount (0.16 wt% - 1.84 wt%), and alcohol/oil molar ratio (6.95:1 - 18.05:1).
All 20 of the designed experiment combinations were conducted, which included eight factorial points, six axial points, and six central points to provide information regarding the interior of the experiment region, allowing for the evaluation of the curvature [16,17].
All experiment combinations were also carried out in triplicate. The mean conversion rate ranged from 65.7% to 97.4 %, depending on the experimental conditions.
3.2.1. Effect of Reaction Time
Under the same experimental conditions, which were: alcohol/oil molar ratio 12:1, catalyst (NaOH) amount 1.0 wt%, reaction temperature 333 K. Figure 3 shows effects of the reaction time for the conversion rate in the second step.
In order to ameliorate the mixing level between alcohol and oil, alkali catalysts were added to the reacting
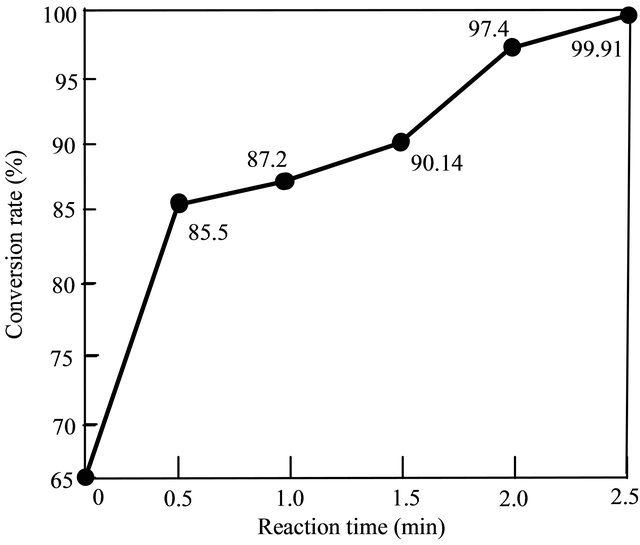
Figure 3. Effects of the reaction time on the conversion rate in the second step.
mixture. Results showed that after 1.5 minutes, the biodiesel conversion rate was more than 90%. The conversion rate reached to a noticeable value of 97.4% when the reaction time was 2.0 minutes. According to the normative standards of biodiesel, the required conversion rate must be 96.4%. Therefore, the best reaction time of the second step should be 2.0 minutes.
In this work, microwave assists the two-step catalyzing process of biodiesel production and the total reaction time required was 9.5 min. The total reaction time were less than previous studies [9,15,18,19] which also transesterification of high FFA content oils with alcohol to biodiesel catalyzed by H2SO4 and NaOH.
3.2.2. Biodiesel Quality
For biodiesel to be used as a motor fuel or blended with petroleum diesel, it must conform to standard specifications. According to the standard of EN-14214, the conversion rate of methyl esters should be 96.4%.
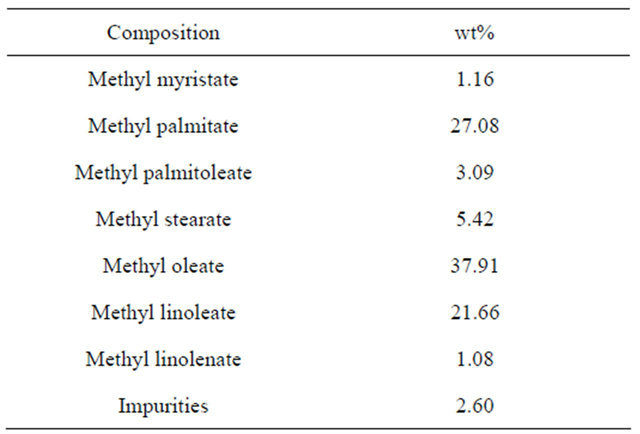
Table 5 shows the gas chromatogram of the biodiesel produced from the waste vegetable oil. The main fatty acid ester constituents included methyl myristate, methyl palmitate, methyl palmitoleate, methyl stearate, methyl oleate, methyl linoleate, and methyl linolenate. In general, saturated fatty acids, e.g. myristic acid (C14) palmitic acid (C16) and stearic acid (C18), have a higher cetane number compared with unsaturated fatty acids which are less susceptible to oxidation.
As a result, the biodiesel produced from the waste vegetable oil, which contains more unsaturated fatty acids, e.g. oleate acid and linoleate acid, is good in colder environment liquidity, but more easily oxidized [20].
4. Conclusions
A two-step catalytic reaction with the assistance of
Table 5. Gas chromatogram of biodiesel from waste vegetable oil in the second step.
co-solvent and microwave to produce of biodiesel from waste vegetable oil was considered in this study. The addition of the co-solvent (THF) effectively increased the efficiency of the reaction. In order to enhance the conversion rate and shorten the reaction time, a two-step catalyzing process was introduced.
The main purpose of the first step was to reduce the acid value to lower than 2.0 KOH mg/g with the following conditions maintained for 7.5 minutes: alcohol to oil molar ratio 9:1, acidic catalyst amount 1 wt%, and reaction temperature 333 K.
In the second step, the biodiesel conversion rate could reach 97.4% with the following conditions maintained for 2.0 minutes: alcohol to oil molar ratio 12:1, alkaline catalyst amount 1 wt%, and reaction temperature 333 K.
The total reaction time was less than previous studies which also transesterification of high FFA content oils using a two-step catalyzing process.
5. Acknowledgements
This study was supported by a research grant from the National Science Council of the Republic of China, grant No. NSC-95-2211-E-168-021.
REFERENCES
- D. E. Lopez, J. G. Goodwin Jr., D. A. Bruce and E. Lotero, “Transesterification of Triacetin with Alcohol on Solid Acid and Base Catalysts,” Applied Catalysis A: General, Vol. 295, No. 2, 2005, pp. 97-105. doi:10.1016/j.apcata.2005.07.055
- Y. Zhang, M. A. Dube, D. D. McLean and M. Kates, “Biodiesel Production from Waste Cooking Oil: 1. Process Design and Technological Assessment,” Bioresource Technology, Vol. 89, No. 1, 2003, pp. 1-16. doi:10.1016/S0960-8524(03)00040-3
- J. M. Marchetti, V. U. Miguel and A. F. Errazu, “Heterogeneous Esterification of Oil with High Amount of Free Fatty Acids,” Fuel, Vol. 86, No. 5, 2007, pp. 906-910. doi:10.1016/j.fuel.2006.09.006
- H. Fukuda, A. Kondo and H. Noda, “Biodiesel Fuel Production by Transesterification of Oils,” Journal of Bioscience and Bioengineering, Vol. 92, No. 5, 2001, pp. 405- 416.
- X. Yuan, J. Liu, G. Zeng, J. Shi, J. Tong and G. Huang, “Optimization of Conversion of Waste Rapeseed Oil with High FFA to Biodiesel Using Response Surface Methodology,” Renewable Energy, Vol. 33, No. 7, 2007, pp. 1678- 1684. doi:10.1016/j.renene.2007.09.007
- A. K. Singh, S. D. Fernando and R. Hernandez, “BaseCatalyzed Fast Transesterification of Soybean Oil Using Ultrasonication,” Energy & Fuels, Vol. 21, No. 2, 2007, pp. 1161-1164. doi:10.1021/ef060507g
- S. Furuta, H. Matsuhashi and K. Arata, “Biodiesel Fuel Production with Solid Amorphous-Zirconia Catalysis in Fixed Bed Reactor,” Biomass & Bioenergy, Vol. 30, No. 10, 2006, pp. 870-873. doi:10.1016/j.biombioe.2005.10.010
- E. Lotero, T. Liu, D. E. Lopez, K. Suwannakarn, D. A. Bruce and J. G. Goodwin Jr., “Synthesis of Biodiesel via Acid Catalysis,” Industrial & Engineering Chemistry Research, Vol. 44, No. 14, 2005, pp. 5353-5363. doi:10.1021/ie049157g
- S. V. Ghadge and H. Raheman, “Biodiesel Production from Mahua (Madhuca Indica) Oil Having High Free Fatty Acids,” Biomass and Bioenergy, Vol. 28, No. 6, 2005, pp. 601-605.
- N. Azcan and A. Danisman, “Alkali Catalyzed Transesterification of Cottonseed Oil by Microwave Irradiation,” Fuel, Vol. 86, No. 17, 2007, pp. 2639-2644. doi:10.1016/j.fuel.2007.05.021
- N. Azcan and A. Danisman, “Microwave Assisted Transesterification of Rapeseed Oil,” Fuel, Vol. 87, No. 10, 2007, pp. 1781-1788. doi:10.1016/j.fuel.2007.12.004
- D. G. B. Boocock, S. K. Konar and H. Sidi, “Phase Diagrams for Oil/Alcohol/Ether Mixtures,” Journal of the American Oil Chemists’ Society, Vol. 73, No. 10, 1996, pp. 1247-1251.
- M.-C. Hsiao, C.-C. Lin, Y.-H. Chang and L.-C. Chen, “Ultrasonic Mixing and Closed Microwave Irradiation Assisted Transesterification of Soybean Oil,” Fuel, Vol. 89, No. 12, 2010, pp. 3618-3622. doi:10.1016/j.fuel.2010.07.044
- G. Vicente, M. Martınez and J. Aracil, “Integrated Biodiesel Production: A Comparison of Different Homogeneous Catalysts Systems,” Bioresource Technology, Vol. 92, No. 3, 2004, pp. 297-305. doi:10.1016/j.biortech.2003.08.014
- Y. Wang, S. Ou, P. Liu, F. Xue and S. Tang, “Comparison of Two Different Processes to Synthesize Biodiesel by Waste Cooking Oil,” Journal of Molecular Catalysis A: Chemical, Vol. 252, No. 1, 2006, pp. 107-112. doi:10.1016/j.molcata.2006.02.047
- G. E. P. Box and N. R. Draper, “Empirical ModelBuilding and Response Surfaces,” John Wiley & Sons, New York, 1986.
- D. C. Montgomery, “Design and Analysis of Experiments,” 3rd Edition, John Wiley & Sons, New York, 1991.
- Y. Liu, L. Wang and Y. Yan, “Biodiesel synthesis Combining Pre-Esterification with Alkali Catalyzed Process Form Rapeseed Oil Deodorizer Distillate,” Fuel Processing Technology, Vol. 90, No. 7, 2009, pp. 857-862. doi:10.1016/j.fuproc.2009.04.005
- X. Deng, Z. Fang and Y.-H. Liu, “Ultrasonic Transesterification of Jatropha curcas L. Oil to Biodiesel by a TwoStep Process,” Energy Conversion and Management, Vol. 51, No. 12, 2010, pp. 2802-2807. doi:10.1016/j.enconman.2010.06.017
- M. Canakci, “The Potential of Restaurant Waste Lipids as Biodiesel Feedstocks,” Bioresource Technology, Vol. 98, No. 1, 2007, pp. 183-190. doi:10.1016/j.biortech.2005.11.022
NOTES
*Corresponding author.

