International Journal of Clinical Medicine
Vol.4 No.5(2013), Article ID:31390,6 pages DOI:10.4236/ijcm.2013.45041
Topical CuradermBEC5 Therapy for Periocular Nonmelanoma Skin Cancers: A Review of Clinical Outcomes
![]()
Australasian Medical Research, Port Vila, Republic of Vanuatu.
Email: bill.cham@gmail.com
Copyright © 2013 Bill E. Cham. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 14th, 2013; revised March 25th, 2013; accepted April 28th, 2013
Keywords: Periocular Skin Cancers; CuradermBEC5; Apoptosis; Solamargine; Basal Cell Carcinoma; Squamous Cell Carcinoma; Cosmesis; Solanum
ABSTRACT
Approximately 5 to 10 percent of all skin cancers occur in the periocular region. Basal cell carcinoma is the most frequent malignant periocular tumor, followed by squamous cell carcinoma, sebaceous gland carcinoma, and malignant melanoma. Nonmelanoma skin tumors at the periocular area often cause disfigurement with destruction of soft conjunctival tissue. Many therapeutic methods have been recommended to combat the morbidity and mortality associated with these lesions. Excisions with frozen-section control or Mohs micrographic surgery are regarded as the gold-standard treatments for periocular basal cell and squamous cell carcinomas. However, these treatment modalities have various limitations and reconstruction surgery is often associated with these treatment options. The chemotherapeutic agents solasodine rhamnosides in a cream formulation CuradermBEC5 are specific, effective and safe treatments for nonmelanoma skin cancers with excellent cosmesis. The antineoplastic mode of action is by apoptosis. In this review it is shown that CuradermBEC5 also treats periocular basal cell carcinoma and squamous cell carcinoma with impressive cosmetic outcomes and no reconstructive surgery is required.
1. Introduction
The two most common forms of periocular skin cancers are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). BCC is a slow-growing, locally invasive epidermal skin tumor that originates in the basal layer of the skin and can cause considerable patient morbidity [1, 2]. BCC is the most common cancer in the white population, and its incidence is increasing at alarming rates. An estimated 2.8 million people in the United States are diagnosed with BCC annually [3,4].
SCC is a malignant tumor that arises from the keratinizing cells of the epidermis or its appendages. It is locally invasive and has the potential to metastasize to other organs of the body.
SCC is the second most common form of skin cancer. An estimated 700,000 cases of SCC are diagnosed each year in the United States [5].
BCC and SCC are collectively referred to as nonmelanoma skin cancers. Over the past three decades, more people have had skin cancer than all other cancers combined [6]. An estimated 3010 deaths from nonmelanoma skin cancers have been predicted to occur in the United States in 2012 [7].
The periocular region is a common site for head and neck skin malignancy. It is bounded by the nose medially, the orbital rim laterally, the brow superiorly and the infraorbital rim inferiorly.
BCC accounts for 80% - 95% and SCC for 5% - 10% of periocular malignancies [8].
Surgical excision and Mohs micrographic surgery are the gold-standard for periocular skin malignancies. Once the tumor has been completely removed, reconstructive surgery is usually necessary.
Reconstruction of periocular defects following excision of cutaneous malignancy can present difficulties for oculofacial and reconstruction surgeons. The intricate anatomy of this area requires precise restoration to avoid postoperative functional and aesthetic concerns. Excision surgery may be very challenging in this anatomical area and can cause cosmetic functional impairment [8]. In addition, not all patients with periocular malignancies are qualified for surgery.
Topical therapy with a cream formulation containing solasodine rhamnosides, CuradermBEC5, produces beneficial end results with a wide range of BCCs and SCCs of varying locations and sizes ranging from millimeters to centimeters [9,10]. Moreover, cosmesis with little or no scar formation with CuradermBEC5 treatment are striking [9-17]. The tissue-sparing technique of CuradermBEC5 also preserves functionality [18].
This communication presents a review of patients with periocular BCCs and SCCs who were treated topically with CuradermBEC5. This anatomical site is considered to be difficult to treat by any modality.
2. Treatments Administered
Selection of study population: Only patients with periocular nonmelanoma skin cancers were selected for this review. Seven patients with BCCs and two patients with SCCs at the periocular region bounded by the nose medially, the orbital rim laterally, the brow superiorly and infraorbital inferiorly were treated topically with CuradermBEC5.
3. BEC-Solasodine Rhamnosides
Glycoalkaloids (BEC) were extracted from the fruit of S. sodomaeum also known as S. linnaeanum (devil’s apple) and S. melongena (eggplant) essentially as described earlier [19]. BEC is a mixture of solasodine glycosides consisting of the triglycosides solasonine (β-solatriose) (33%), solamargine (β-chacotriose) (33%), and di-and-monoglycosides (34%). All the glycosides contain the same aglycone solasodine [19-24].
4. CuradermBEC5 Topical Cream Formulation
The cream formulation CuradermBEC5 is available to patients in several countries. CuradermBEC5 contains the glycoalkaloids BEC at 0.005% as a topical formulation [9,10].
5. CuradermBEC5 Treatment Procedure [25]
The patients were instructed to use CuradermBEC5 as follows:
• Wash the lesion and the surrounding area with a mild non-irritating soap;
• Rinse with water;
• Dry thoroughly;
• Unscrew the lid of the CuradermBEC5 tube and remove the protective foil that covers the hole in the lid of the tube;
• Apply CuradermBEC5 to the lesion, just enough to cover the lesion. Spread evenly over lesion only. Do not apply the cream in large quantity and do not extend the cream more than 0.5 cm on the apparently normal skin surrounding the edge of the lesion;
• Apply the cream to the lesion by gently squeezing the tube;
• Cover each lesion with an occlusive dressing (for example paper tape) until the next application of CuradermBEC5;
• Apply the cream to the lesion twice daily, i.e. every 12 hours;
• Stop treatment only when the lesion has been completely cleared and replaced with normal skin.
6. Results
Histological analyses of biopsies confirmed the clinical evaluations. In some cases biopsies were taken after completion of treatment. Some patients refused to have biopsies taken after treatment for cosmetic reasons. Nevertheless, all patients, except one, which is currently being monitored, were followed-up for over 5 years. All showed no recurrence of their treated lesions.
Figures 1 and 2 show clinical and histological evaluations of patients who were treated with CuradermBEC5 for periocular SCCs.
Figures 3 to 9 illustrate periocular BCCs that were treated with CuradermBEC5.
The general observed pattern of response with CuradermBEC5 therapy entailed swelling, erythema, erosion,
 (a)
(a)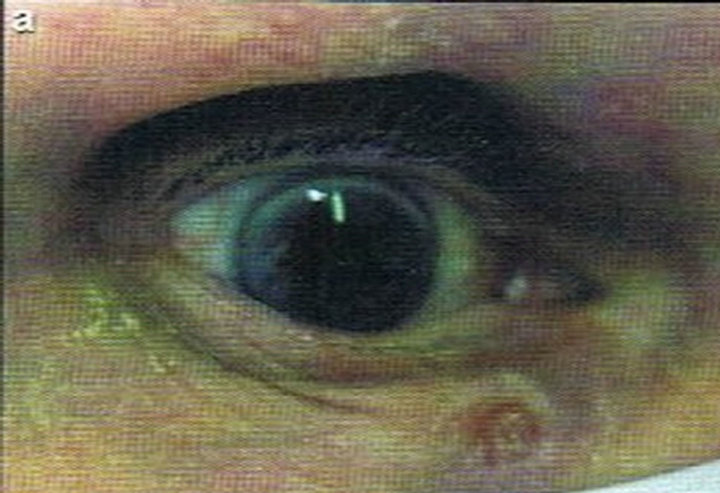 (b)
(b)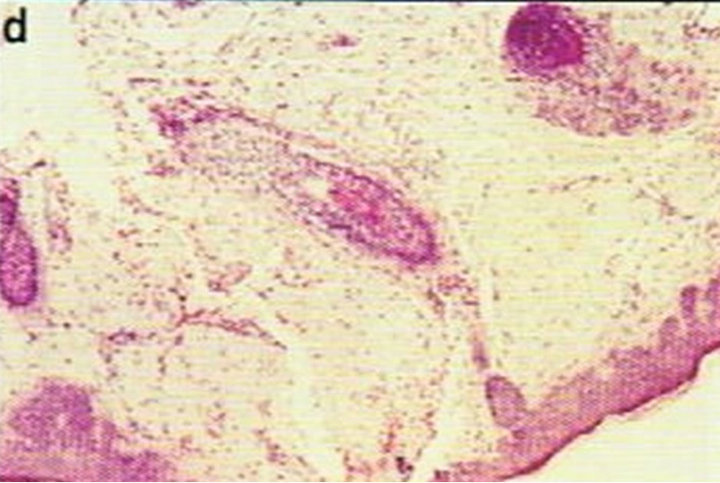 (c)
(c)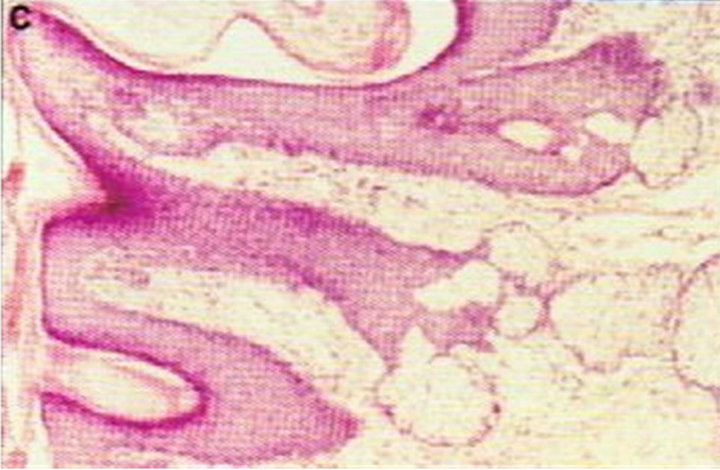 (d)
(d)
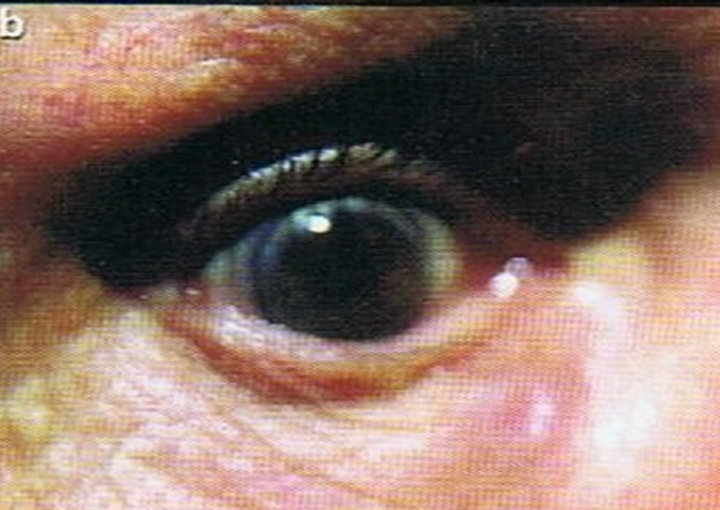
Figure 1. Periocular SCC under the right eye (infraorbital rim) of a patient before (a) and after (b) CuradermBEC5 treatment. After treatment there was no trace of the SCC. Confirmation by histological analyses of the SCC before treatment (c) and after treatment (d) are shown. The total treatment period was 14 weeks. Clinical assessment 5 years post treatment revealed that there was no recurrence [16].
 (a)
(a)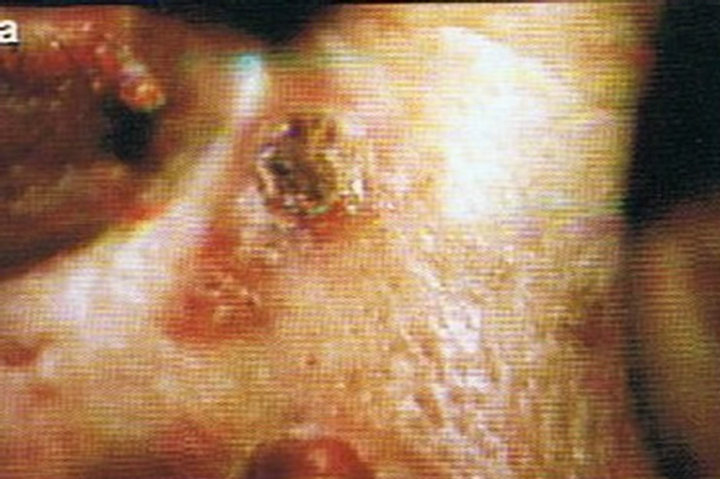 (b)
(b)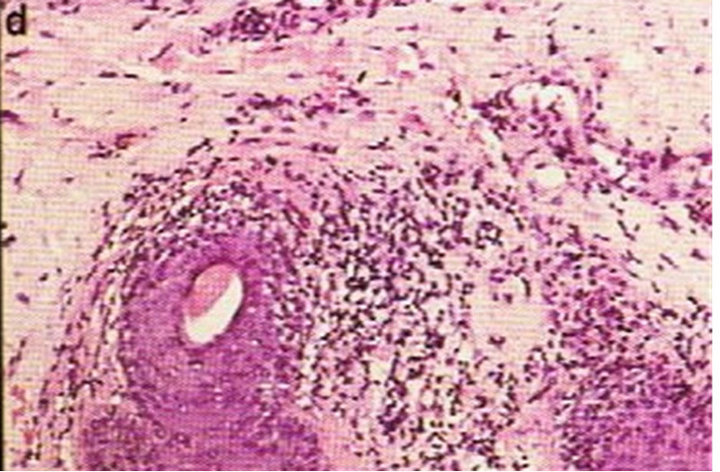 (c)
(c)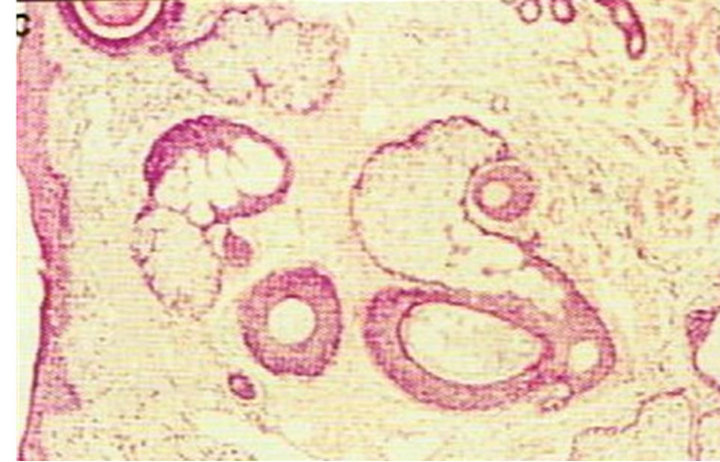 (d)
(d)
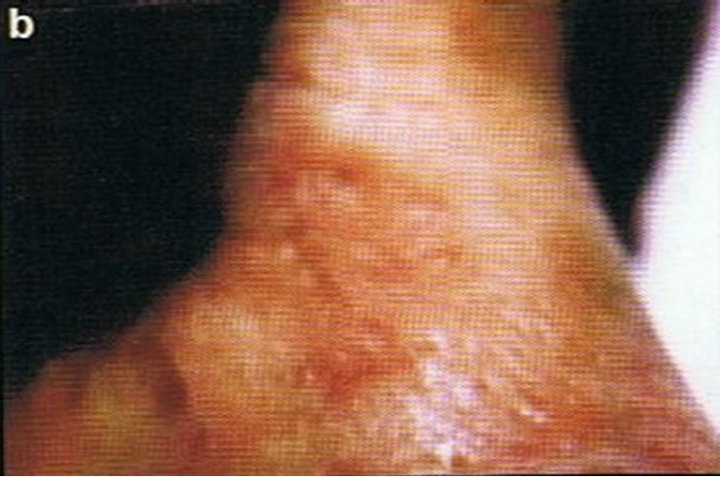
Figure 2. Periocular SCC medial to the nose (a). This SCC was starting to impair the vision of the patient. After CuradermBEC5 therapy the lesion was cleared (b). After completion of treatment the vision was restored. Treatment duration was 10 weeks. The clinical diagnosis was confirmed histologically by punch biopsy (c). After completion of CuradermBEC5 therapy, histopathology determined that no residual cancer was present (d). Clinical assessment 5 years post treatment revealed that there was no recurrence [16].
 (a)
(a)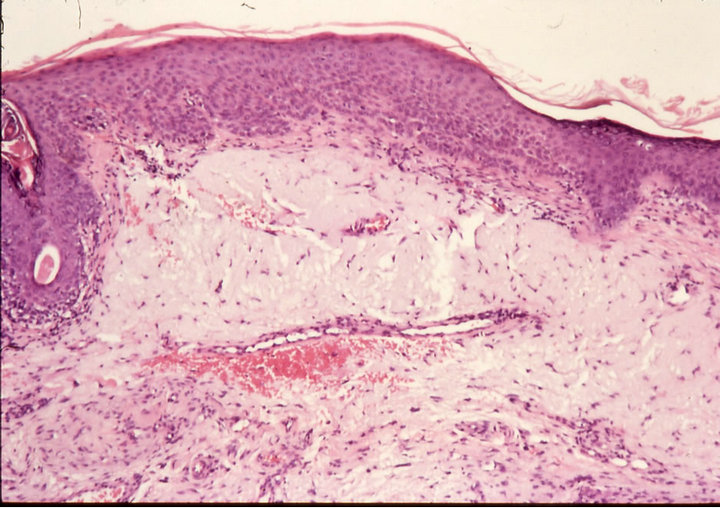 (b)
(b)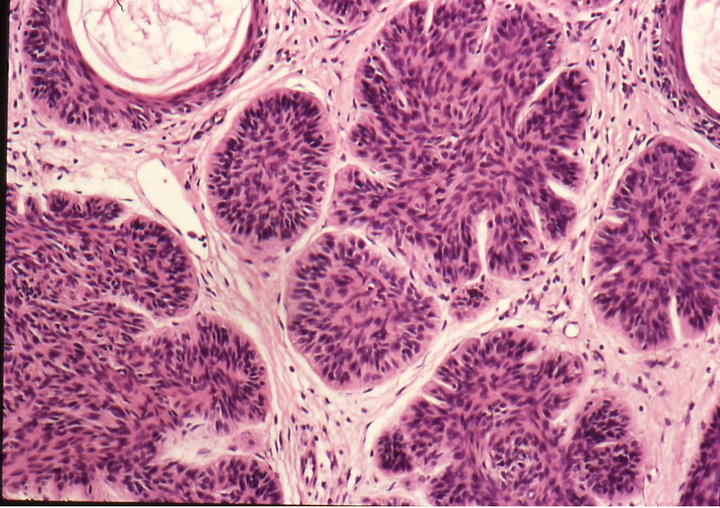 (c)
(c)
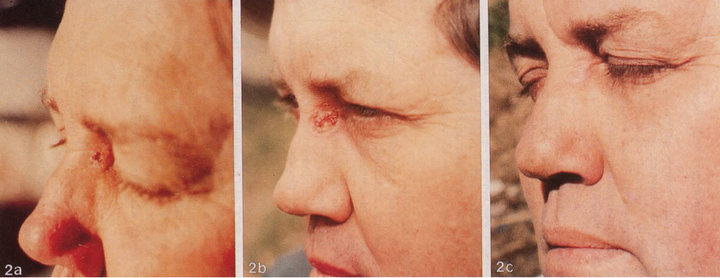
Figure 3. Clinical progress of a periocular BCC on a patient before treatment (a), two weeks after commencement of therapy (b), and site of treated BCC after completion of therapy with CuradermBEC5 (c). Treatment period was 5 weeks. Histological analyses before (d) and after (e) therapy show that the periocular BCC was cleared with CuradermBEC5 therapy. There was no recurrence after 5 years [13].
ulceration and regrowth of normal tissue. Tingling, or some pain was experienced for 15 to 30 minutes after each application. In the initial stages of CuradermBEC5 therapy, the treated lesion increased in size. From a clini-
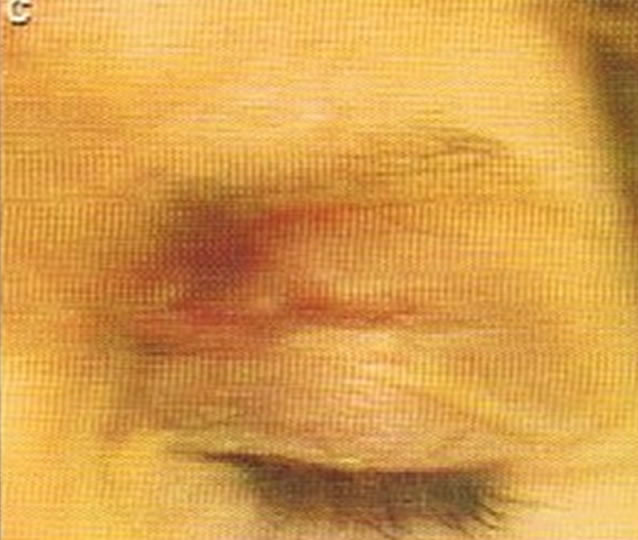 (a)
(a)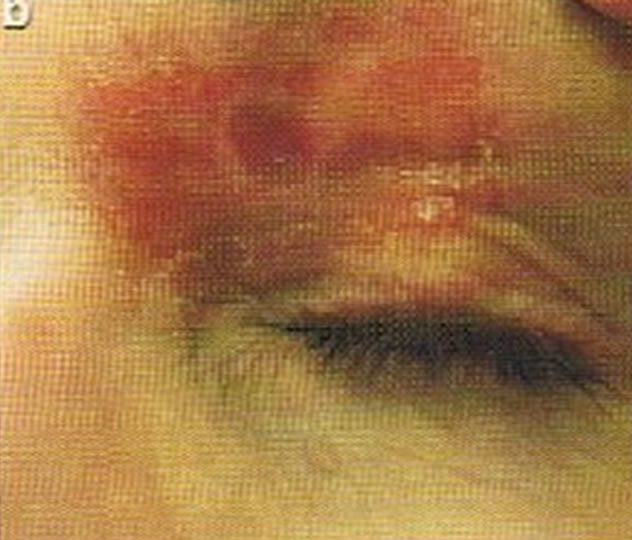 (b)
(b)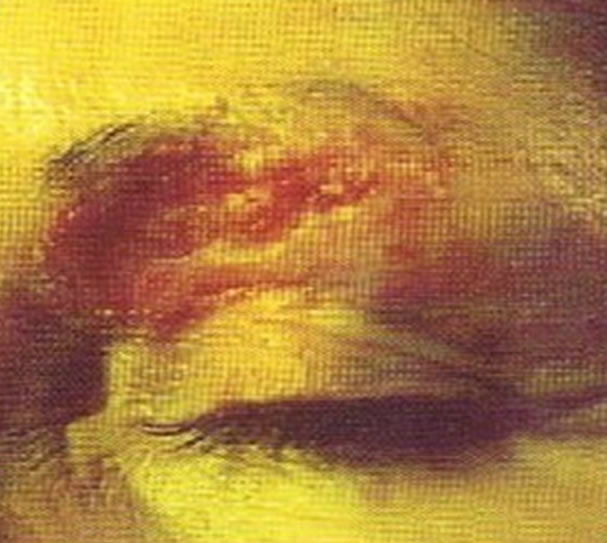 (c)
(c)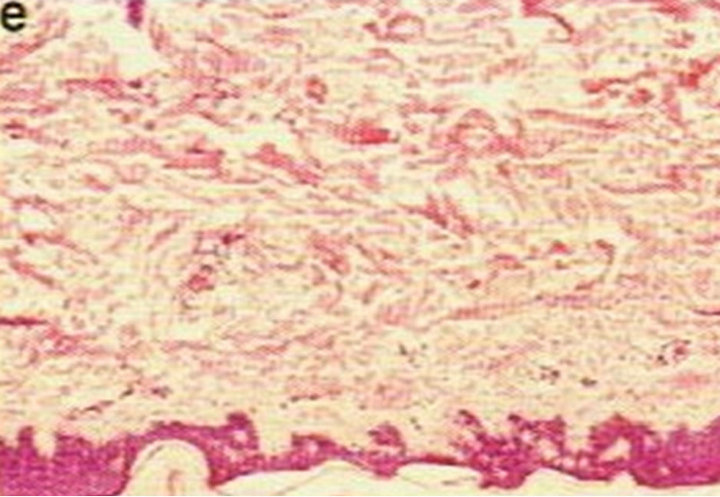 (d)
(d)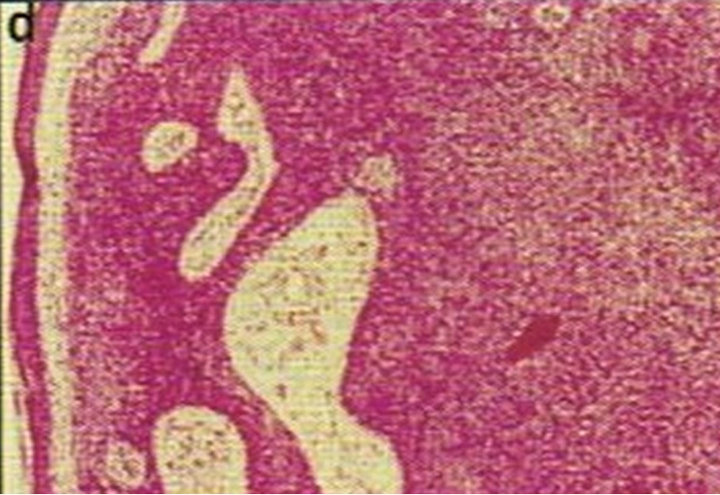 (e)
(e)
Figure 4. Periocular BCC, superior to the eye including part of the brow, of a patient before (a), during (b) and after (c) CuradermBEC5 therapy. During CuradermBEC5 treatment the lesion was much smaller and residual tumor can distinctly be seen which was surrounded by some inflammation. After treatment there was no sign of the BCC. Confirmation by histological period was 9 weeks. Clinical assessment 5 years post treatment showed that there was no recurrence [16].
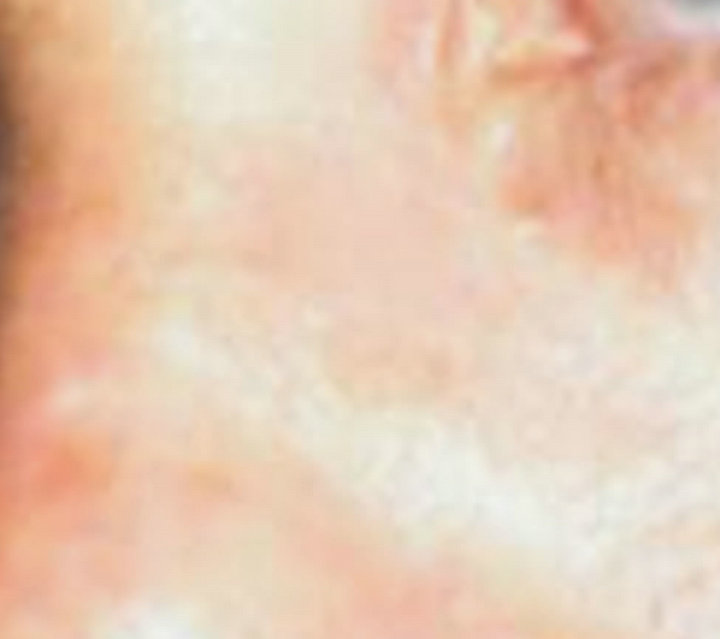 (a)
(a)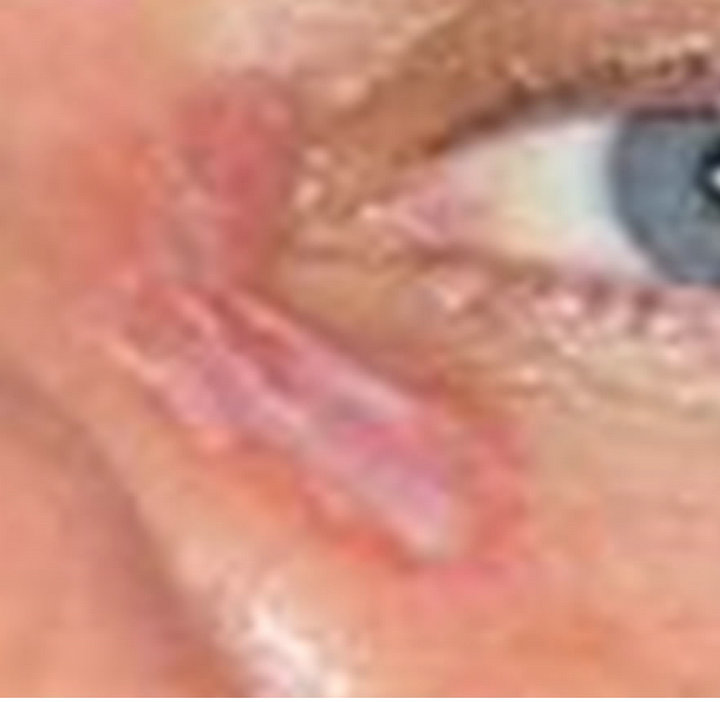 (b)
(b)
Figure 5. Periocular BCC before (a) and after (b) CuradermBEC5 therapy. Treatment period was 8 weeks. There was no recurrence after 5 years [25].
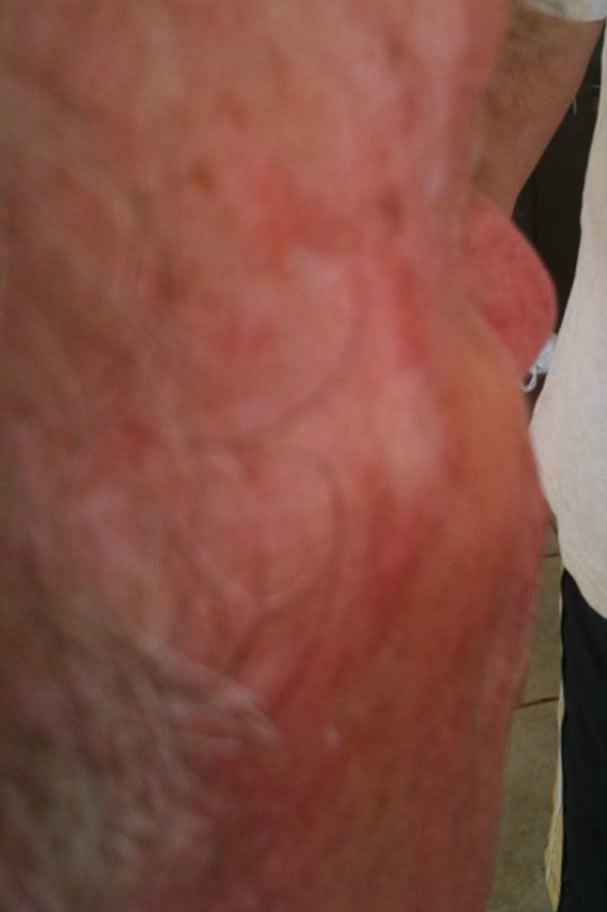 (a)
(a)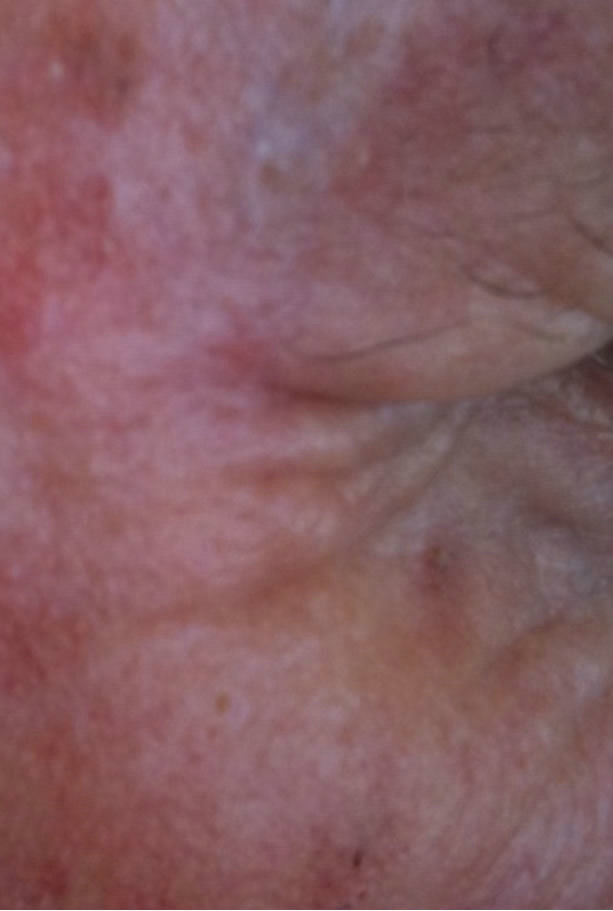 (b)
(b)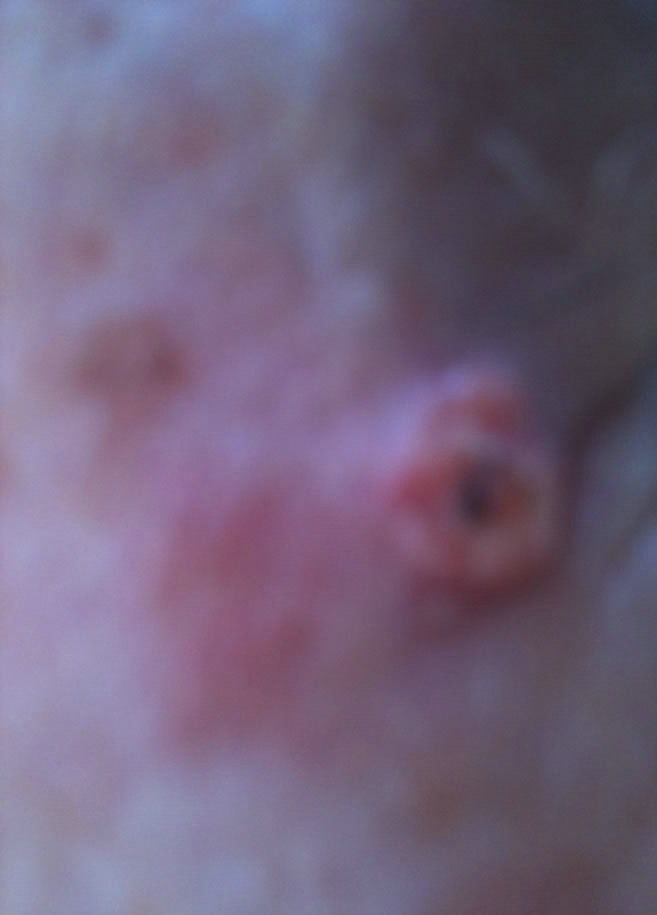 (c)
(c)
Figure 6. Periocular BCC. Before (a) and after (b) and (c) CuradermBEC5 therapy. The BCC protruded from the skin before treatment. After treatment it was not possible to distinguish where the cancer was prior to treatment. Treatment period was 6 weeks. There was no recurrence after 5 years [25].
 (a)
(a)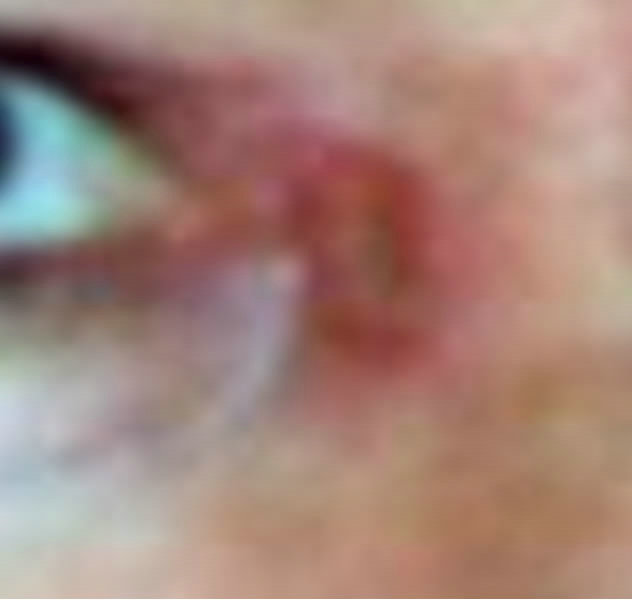 (b)
(b)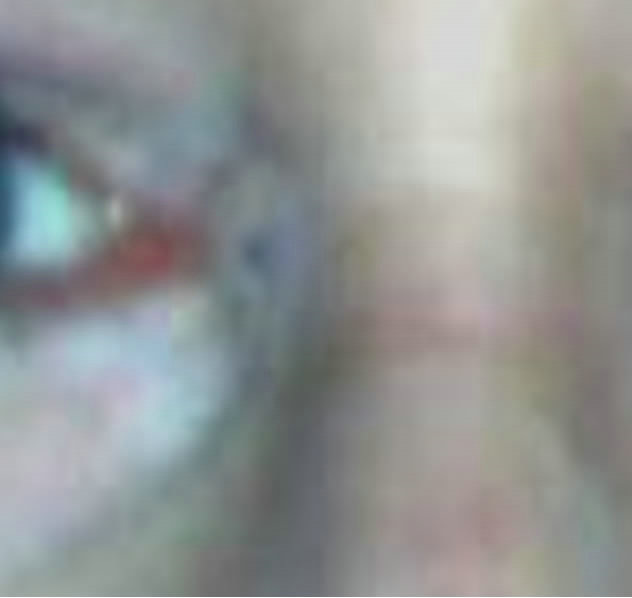 (c)
(c)
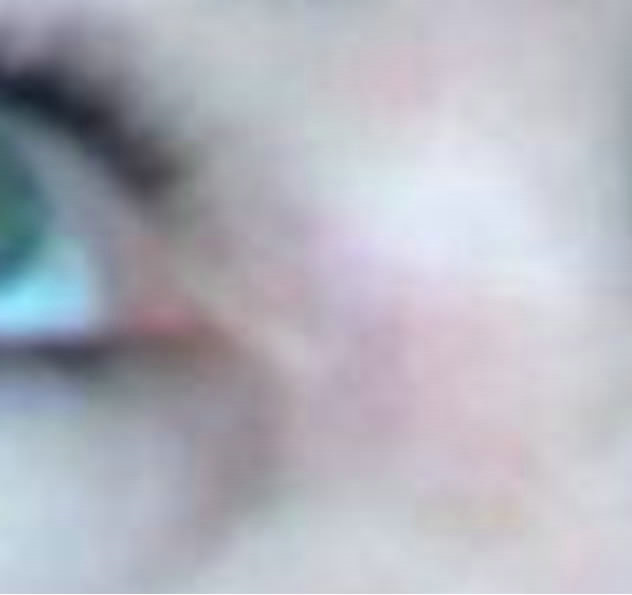
Figure 7. Periocular BCC medial to the nose. Before (a), during (b) and after (c) CuradermBEC5 therapy. Treatment period was 5 weeks. There was no recurrence after 5 years [25].
 (a)
(a)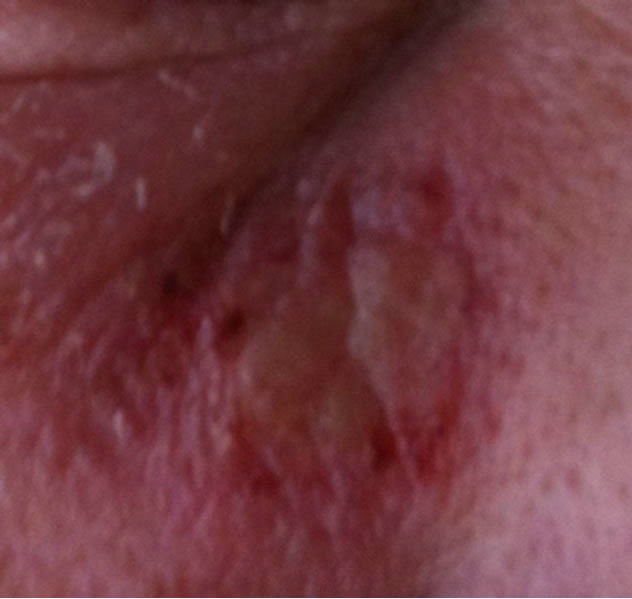 (b)
(b)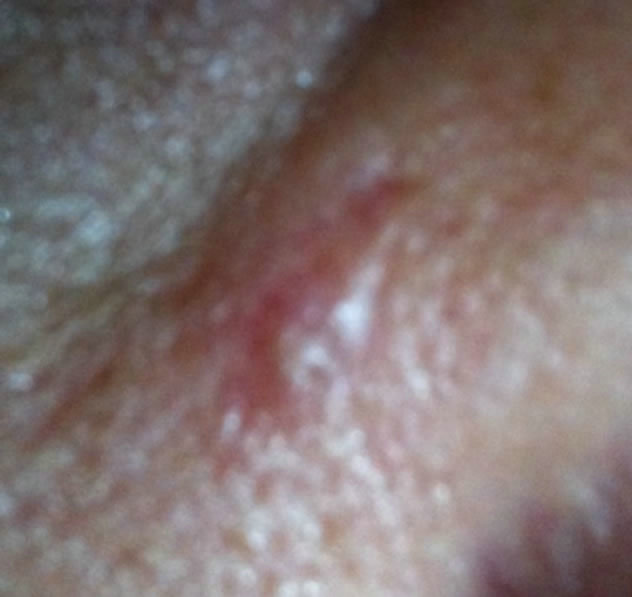 (c)
(c)
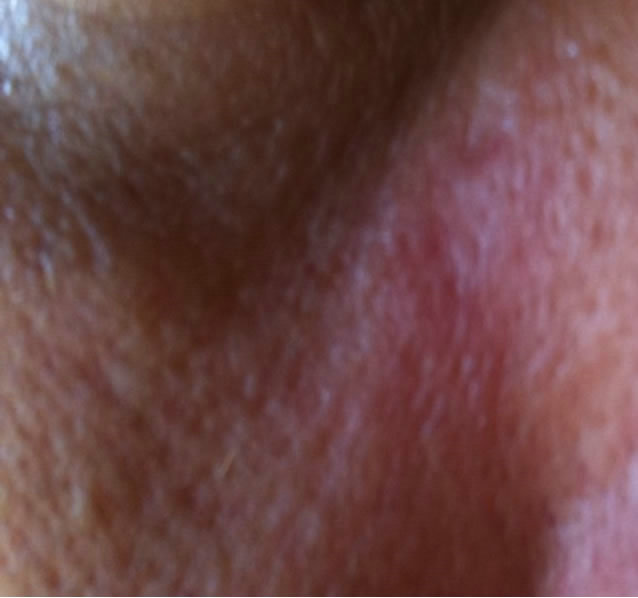
Figure 8. Periocular BCC at the infraorbital rim of a patient. Before (a), during (b) and after (c) treatment with CuradermBEC5. Treatment period was 4 weeks. There was no recurrence after 5 years [25].
 (a)
(a)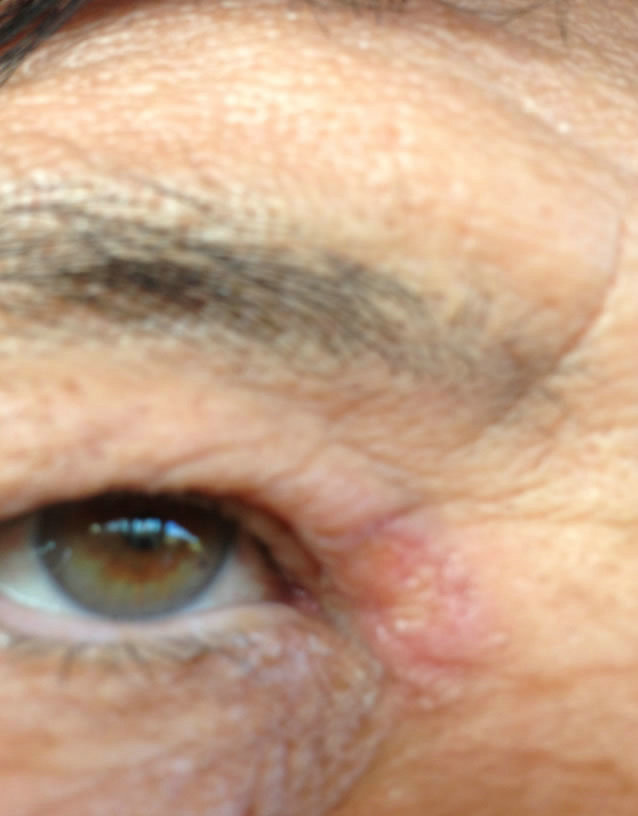 (b)
(b)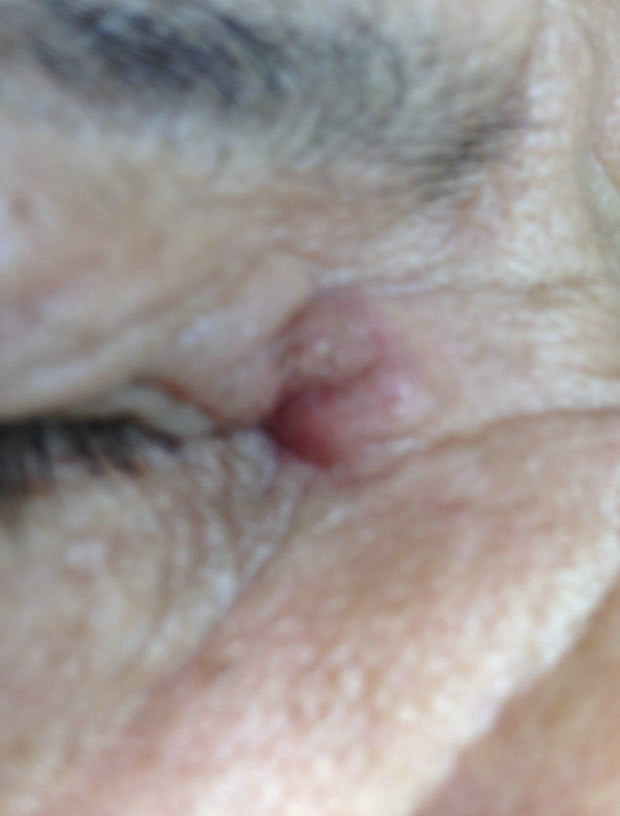 (c)
(c)
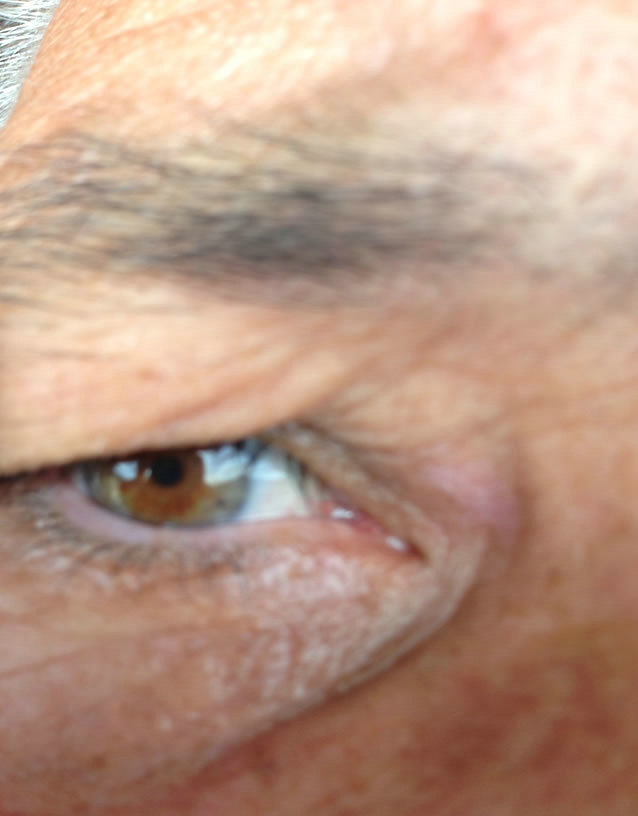
Figure 9. Periocular BCC medial to the nose. Before (a), during (b) and after (c) CuradermBEC5 treatment. Treatment period was 5 days. Still being followed-up [25].
cal perspective, the increase in size seemed to correlate with the extent of tumor presence at the lesion site. During this stage some patients experienced pain or a burning sensation for 15 to 30 minutes after application of the cream. After sometime during treatment, the lesion started to reduce in size. Treatment was continued until the lesion was completely cleared and was replaced with normal skin. At the end of treatment some erythema was observed which lasted several days.
7. Discussion
Skin cancer falls into two major groups, nonmelanoma and melanoma. BCC and SCC are types of nonmelanoma skin cancers and are the most common skin cancers. BCC rarely metastasizes or kills. However, it can cause significant destruction, disfigurement and loss of functionality of the affected area. SCCs are locally invasive and can metastasize with potential fatal sequelae.
In the United States approximately 3 out of 10 and in Australia 1 out of 2 Caucasians may develop BCC within their lifetime. In 80% of all cases, BCCs are found on the head and neck [6,7].
The treatment for these nonmelanoma skin cancers depends on their type, size and location, the number to be treated, and the preference or expertise of the doctor.
It has been documented that the most effective treatment option is by surgical excision. However, it has been reported that the recurrence rates using this procedure can be very high, ranging from 30 to 67 percent [26].
Over the last few decades non-surgical treatments have become available. These include cryotherapy, topical fluorouracil and imiquimod creams, radiotherapy and photodynamic therapy. These procedures have many beneficial outcomes when treating early-detected nonmelanoma skin cancers. However, there are also many limitations and some disadvantages using these techniques are: requirement of local anesthetics, procedural complications, discomfort and/or pain, multiple visits to the doctor, possible infection, risk of scarring, disfigurement, long treatment periods, changes in pigmentation, high recurrence rates and possible requirement of reconstruction after treatment [25].
Surgical excision and Mohs micrographic surgery are the most effective treatments for periocular skin cancers. Reconstruction of the resulting defect is tailored to preserve function, protect the eye, and provide a satisfactory cosmetic appearance. Mohs micrographic surgery procedure may become tedious, prolonged and exhausting for the patient; especially if the case is difficult or complex as is the case with periocular tumors. This procedure requires a specially trained dermatologist and ancillary staff. Multiple injections of local anesthetic can cause discomfort for the patient. Mohs treatment fails in tumors that have satellitosis, a multicentric origin, or skip areas. Surgical procedures are also costly.
Unfortunately, not all patients with periocular malignancies qualify for surgical intervention and many patients find it daunting to have surgical procedures around the eyes. Consequently the lesion grows larger with potentially drastic sequelae.
This current communication shows clearly that topical treatments of periocular BCCs and SCCs with Curaderm BEC5 result in impressive clinical outcomes.
The antineoplastic mode of action of the solasodine rhamnosides, solamargine and solasonine, present in CuradermBEC5 may explain the remarkable observed clinical outcomes. Specific endogenous endocytic lectins (EELs) have been identified on cancer cells [27]. These EELs have been further characterized as rhamnose binding protein (RBP) receptors [28]. RBP receptors are present on cancer cells but not normal cells [27,28]. RBP receptors bind the solasodine rhamnosides (BEC). BEC is then internalized into the cancer cells by receptor-mediated endocytosis through “coated pit endocytosis”. BEC interacts with the lysosomes and mitochondria resulting in the triggering of extrinsic and intrinsic apoptotic pathways in the cancer cells by up-regulating the expression of external death receptors, such as tumor necrosis factor receptor 1 (TNFR-1), Fas receptor, TNFR-1 associated death domain and Fas-associated death domain [29,30]. BEC enhances the intrinsic ratio of Bax to Bcl-2 by upregulating Bax and down-regulating Bcl-2 and Bcl-x expressions. These effects result in activation of Caspase-8, -9 and -3 in cancer cells [24-34], indicating that BEC triggers extrinsic and intrinsic apoptotic pathways in cancer cells and causes apoptosis to cancer cells.
These events may explain the clinical observations that treatment with CuradermBEC5 results in elimination of cancer cells only and not normal cells. Very importantly, whilst cancer cells are being destroyed by CuradermBEC5, normal cells are replenishing the dead cancer cells and this exceptional occurrence translates to the observed cosmesis effects of CuradermBEC5 therapy. Moreover, CuradermBEC5 therapy clears cancer cells whether they are proliferating or not [25].
Clinical observations with CuradermBEC5 therapy reveal that initially the lesion size increases significantly due to interaction of CuradermBEC5 with deeper seated and more lateral tumor cells [15-17,25]. As treatment progresses, the size of the lesion decreases due to the elimination of CuradermBEC5 affected cancer cells, which are replaced with normal skin cells. Less CuradermBEC5 cream is then applied to the smaller sized lesion until the lesion is completely cleared and replaced with normal skin cells [25].
The pain during treatment experienced by some patients may be explained by the keratolytic agents salicylic acid and urea and not BEC as was previously reported in placebo controlled clinical trials with Curaderm BEC5 [11,35,36].
CuradermBEC therapy is stopped only after the lesion has been completely replaced with normal tissue. This explains why treatment periods of CuradermBEC5 therapy vary and are dependent on size, location and type of tumor tissue.
These striking observations with CuradermBEC5 therapy are vastly different than all other therapies that are used for treatment of neoplastic cells. After Curaderm BEC5 therapy, no reconstructive surgery is required. The body heals itself with no disfigurement, and, functionality of the tissue is preserved [18].
8. Conclusion
CuradermBEC5 therapy for periocular nonmelanoma skin cancers is very effective and safe. The treatment of these lesions overcomes the major drawbacks of other currently available therapies. CuradermBEC5 is specific and eliminates the cancer cells only, without harming normal cells and consequently cosmesis is excellent. No reconstructive surgery is required with CuradermBEC5 therapy. These preliminary observations warrant more extensive clinical evaluations to determine the potential of this treatment modality for periocular skin cancers.
REFERENCES
- S. J. Miller, “Biology of Basal Cell Carcinoma (Part 1),” Journal of the American Academy of Dermatology, Vol. 24, No. 1, 1991, pp. 1-13.
- A. N. Crowson, “Basal Cell Carcinoma: Biology, Morphology and Clinical Implications,” Modern Pathology, Vol. 19, No. S2, 2006, pp. 5127-5147. doi:10.1038/modpathol.3800512
- D. L. Miller and M. A. Weinstock, “Nonmelanoma Skin Cancer in the United States: Incidence,” Journal of the American Academy of Dermatology, Vol. 30, No. 5, 1994, pp. 774-778.
- D. H. Brewster, L. A. Bhatti, J. H. C. Inglis, E. R. Nairn and V. R. Doherty, “Recent Trends in Incidence of Nonmelanoma Skin Cancers in the East of Scotland, 1992- 2003,” British Journal of Dermatology, Vol. 156, No. 6, 2007, pp. 1295-1300. doi:10.1111/j.1365-2133.2007.07892.x
- American Academy of Dermatology, “Squamous Cell Carcinoma,” 2012. http://www.aad.org/skin-conditions/dermatology-a-to-z/squamous-cell-carcinoma
- R. S. Stern, “Prevalence of a History of Skin Cancer in 2007: Results of an Incidence-Based Model,” Archives Dermatology, Vol. 146, No. 3, 2010, pp. 279-282. doi:10.1001/archdermatol.2010.4
- American Cancer Society, “Cancer Facts & Figures,” 2012.
- R. I. Ceilley and J. Q. del Rosso, “Current Modalities and New Advances in the Treatment of Basal Cell Carcinoma,” International Journal of Dermatology, Vol. 45, No. 5, 2006, pp. 489-498. doi:10.1111/j.1365-4632.2006.02673.x
- B. E. Cham, “Intralesion and Curaderm BEC5 Topical Combination Therapies of Solasodine Rhamnosyl Glycosides Derived from the Eggplant or Devil’s Apple Result in Rapid Removal of Large Skin Cancers. Methods of Treatment Compared,” International Journal Clinical Medicine, Vol. 3, No. 2, 2012, pp. 115-124. doi:10.4236/ijcm.2012.32024
- B. E. Cham, “Topical Solasodine Rhamnosyl Glycosides Derived from the Eggplant Treats Large Skin Cancers: Two Case Reports,” International Journal Clinical Medicine, Vol. 2, No. 4, 2011, pp. 473-477. doi:10.4236/ijcm.2011.24080
- S. Punjabi, L. J. Cook, P. Kersey, R. Marks and R. Cerio, “Solasodine Glycoalkaloids: A Novel Topical Therapy for Basal Cell Carcinoma. A Double-Blind, Randomized, Placebo-Controlled, Parallel Group, Multicentre Study,” International Journal Dermatology, Vol. 47, 2008, pp. 78-82. doi:10.1111/j.1365-4632.2007.03363.x
- B. E. Cham and H. M. Meares, “Glycoalkaloids from Solanum sodomaeum L. Are Effective in the Treatment of Skin Cancers in Man,” Cancer Letters, Vol. 36, No. 2, 1987, pp. 111-118. doi:10.1016/0304-3835(87)90081-4
- B. E. Cham, B. Daunter and R. Evans, “Topical Treatment of Malignant and Premalignant Skin Cancers by Very Low Concentrations of a Standard Mixture of Solasodine Glycosides,” Cancer Letters, Vol. 59, No. 3, 1991, pp. 183-192. doi:10.1016/0304-3835(91)90140-D
- B. E. Cham, “Solasodine Glycosides as Anti-Cancer Agents: Pre-Clinical and Clinical Studies,” Asia Pacific Journal Pharmacology, Vol. 9, No. 2, 1994, pp. 113-118.
- B. E. Cham, “Solasodine Rhamnosyl Glycosides in a Cream Formulation Is Effective for Treating Large and Troublesome Skin Cancers,” Research Journal Biological Science, Vol. 2, No. 7, 2007, pp. 749-761.
- B. E. Cham, “Solasodine Rhamnosyl Glycosides Specifically Bind Cancer Cell Receptors and Induce Apoptosis and Necrosis. Treatment for Skin Cancer and Hope for Internal Cancers,” Research Journal Biological Science, Vol. 2, No. 7, 2007, pp. 503-514.
- T. R. Chase, “CuradermBEC5 for Skin Cancers, Is It? An Overview,” Journal Cancer Therapy, Vol. 2, No. 5, 2011, pp. 728-745. doi:10.4236/jct.2011.25099
- L. H. Goldberg, J. M. Landau, M. N. Moody and I. J. Vergilis-Kalner, “Treatment of Bowen’s Disease on the Penis with Low Concentration of a Standard Mixture of Solasodine Glycosides and Liquid Nitrogen,” Dermatologic Surgery, Vol. 37, 2011, pp. 858-861. doi:10.1111/j.1524-4725.2011.02014.x
- B. E. Cham and L. Wilson, “HPLC of Glycoalkaloids from Solanum sodomaeum,” Planta Medica, Vol. 53, No. 1, 1987, pp. 34-36. doi:10.1055/s-2006-962612
- B. E. Cham, M. Gilliver and L. Wilson, “Antitumour Effects of Glycoalkaloids Isolated from Solanum sodomaeum L.,” Planta Medica, Vol. 53, No. 1, 1987, pp. 34- 36. doi:10.1055/s-2006-962612
- B. E. Cham and B. Daunter, “Solasodine Glycosides. Selective Cytotoxicity for Cancer Cells and Inhibition of Cytotoxicity by Rhamnose in Mice with Sarcoma 180,” Cancer Letters, Vol. 55, 1990, pp. 221-225. doi:10.1016/0304-3835(90)90122-E
- B. E. Cham and T. R. Chase, “Solasodine Rhamnosyl Glycosides Cause Apoptosis in Cancer Cells, Do They Also Prime the Immune System Resulting in Long Term Protection against Cancer?” Planta Medica, Vol. 78, 2012, pp. 349-353. doi:10.1055/s-0031-1298149
- B. E. Cham, “Monograph on the Compound BEC,” Drugs of the Future, Vol. 13, 1988, pp. 714-716.
- B. E. Cham, “The Eggplant Cancer Cure. A Treatment for Skin Cancer and New Hope for Other Cancers from Nature’s Pharmacy,” Smart Publications, Petaluma, 2007, pp. 1-122.
- B. E. Cham, “Inspired by Nature, Proven by Science. The New Generation Cancer Treatment That Causes Cancer Cells to Commit Suicide,” in Preparation.
- L. A. E. Sussman and D. F. Liggins, “Incompletely Excised Basal Cell Carcinoma a Management Dilemma?” Aust NZ Journal Surgery, Vol. 66, 1996, pp. 276-278. doi:10.1111/j.1445-2197.1996.tb01184.x
- B. Daunter and B. E. Cham, “Solasodine Glycosides, in Vitro Preferential Cytotoxicity for Human Cancer Cells,” Cancer Letters, Vol. 55, No. 3, 1990, pp. 209-220. doi:10.1016/0304-3835(90)90121-D
- R. J. Lipscombe, S. J. Carter and M. Ruane, “Rhamnose Binding Protein,” United States Patent 6, 930, 171 B2, 2005.
- L. Y. Shiu, L. C. Chang, C. H. Liang, Y. S. Huang, H. M. Sheu and K. W. Kuo, “Solamargine Induces Apoptosis and Sensitizes Breast Cancer Cells to Cisplatin,” Food Chemical Toxicology, Vol. 45, No. 11, 2007, pp. 2155- 2164. doi:10.1016/j.fct.2007.05.009
- C. H. Liang, L. Y. Shiu, L. C. Chang, H. M. Sheu and K. W. Kuo, “Solamargine Upregulation of Fas, Downregulation of HER 2, and Enhancement of Cytotoxicity Using Epirubicin in NSCLC Cells,” Molecular Nutrition Food Research, Vol. 51, 2007, pp. 999-1005. doi:10.1002/mnfr.200700044
- L. Y. Shiu, C. H. Liang, L. C. Chang, H. M. Sheu, E. M. Tsai and K. W. Kuo, “Solamargine Induces Apoptosis and Enhances Susceptibility to Trastazumab and Epirubicin in Breast Cancer Cells with Low or High Expression Levels of HER2/neu,”Bioscience Reports, Vol. 29, No. 1, 2009, pp. 35-45. doi:10.1042/BSR20080028
- L. Sun, Y. Zhao, X. Li, H. Yuan, A. Cheng and H. Lou, “A Lysosomal-Mitochondrial Death Pathway Is Induced by Solamargine in Human K562 Leukemia Cells,” Toxicology in Vitro, Vol. 24, No. 6, 2010, pp. 1504-1511. doi:10.1016/j.tiv.2010.07.013
- L. Sun, Y. Zhao, H. Yuan, X. Li, A. Cheng and H. Lou, “Solamargine, a Steroidal Alkaloid Glycoside, Induces Oncosis in Human K562 Leukemia and Squamous Cell Carcinoma KB Cells,” Cancer Chemotherapy Pharmacology, Vol. 65, No. 4, 2010, pp. 1125-1130.
- X. Li, Y. Zhao, W. K. K. Wu, S. Liu, M. Cui and H. Lou, “Solamargine Induces Apoptosis Associated with p53 Transcription-Dependent and Transcription-Independent Pathways in Human Osteosarcoma U20S Cells,” Life Science, Vol. 88, No. 7-8, 2011, pp. 314-321.
- B. E. Cham, “Solasodine Glycosides: A Topical Therapy for Actinic Keratosis. A Single-Blind, Randomized, Placebo-Controlled, Parallel Group Study,” Journal Cancer Therapy, Vol. 4, No. 2, 2013, pp. 588-596. doi:10.4236/jct.2013.42076
- B. E. Cham, “Drug Therapy: Solamargine and Other Solasodine Rhamnosyl Glycosides as Anticancer Agents,” Modern Chemotherapy, Vol. 2, No. 2, 2013, pp. 33-49. doi:10.4236/mc.2013.22005

