Natural Resources
Vol.06 No.10(2015), Article ID:60556,10 pages
10.4236/nr.2015.610048
Kinetic Study of Curing Phenol-Formaldehyde/ Tannin-Formaldehyde Composite Resins
Hussein Ali Shnawa1*, Ibraheem Kadum Ibraheem1, Ashwaq Aboud Shenta2
1Polymer Research Center, University of Basrah, Basrah, Iraq
2Department of Chemistry, College of Education for Pure Science, University of Basrah, Basrah, Iraq
Email: *hussanqi@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 10 April 2015; accepted 20 October 2015; published 23 October 2015
ABSTRACT
This work presents a study on the uses of tannin-formadehyde derivative into phenolic resins. Eucalyptus tannins (T) were reacted with formaldehyde to form tannin-formaldehyde resin (TF). Then this derivative was used to prepare tannin-formaldehyde/phenol-formaldehyde resins (TFPF) at 20 and 40 %w/w. The kinetic values of thermal curing of Phenol-formaldehyde (PF), tannin-formaldehyde and tannin-formaldehyde/phenol-formaldehyde resins (TFPF) at 20 and 40 wt% from TF were studied by monitoring the weight changes which occurred in the samples weight during thermosetting process at four temperature (160˚C, 180˚C, 200˚C and 220˚C). The total evolved condensation products from curing reactions were about 32% - 36% per sample weight, and the rate of curing reaction constants was ranged between 0.163 %wt・min−1 at 160˚C and 0.50 %wt・min−1 at 220˚C. The path of TFPF curing and kinetic values indicated that these resins could be cured with the behavior and velocity comparable to that of PF. The activation energy of TFPF cross-linking was higher than that of PF. Increasing TF level to 20% and 40% into PF can reduce the amount of PF curing reactions density and weight loss percentage. The global kinetic properties showed that the TF participated in the thermoset network formation with acceptable activity and performance. The general results of this paper show that the TF is a suitable alternative material for partially replacement into PF resin.
Keywords:
Phenol-Formaldehyde, Tannin-Formaldehyde, Curing Reactions, Weight Loss Monitoring, Kinetic Properties

1. Introduction
Phenol-formaldehyde (PF) resins are one of the oldest synthetics thermoset resins. They were derived from the reaction of phenol or mixture of phenols with formaldehyde [1] . Generally, there are two types from PF resins: Novolak and Resols, the first one, produced under acidic conditions, while the other manufactured by alkaline catalyzed reactions of phenol with excess amount from formaldehyde. The reaction groups in PF resin are methylol groups (-CH2OH) which are chemically reactive functional groups. They can undergo polymerization and curing reaction with itself or with other compounds under usual conditions [2] . One of the negative and limited factors for uses of PF resin was the high cost of phenols which was dependent on petroleum based raw materials.
Due to the high consumption of PF as adhesives for wood industries; many attempts and studies and projects have been focused on fractionally replacement of these resins by natural and more environmental friendly materials such as tannins or other natural phenolic materials. Tannins as bio phenolic product have chemical and physical properties to act as extenders or phenolic based adhesives [3] - [6] . In addition to the natural availability of tannins, the phenolic structure of tannins gives relatively high reactivity to the reaction with formaldehyde to form methylolic resins [6] . In his study, Herbert [7] reported that about 30% - 50% of amino or phenolic resins can be replaced by tannins into wood adhesives. Saayman [8] reported that wattle tannin in resol- type PF for bonding wood veneers required up to 30% PF fortification to obtain a fully water-resistant bond.
Pizzi [9] reviewed the current status of technology on phenolic and tannin adhesives for wood panel products, while Berg [10] and his coworkers reviewed Chilean efforts in utilizing tannins (pinus radiata bark extracts) adhesives for commercial particle board panels. Sellers Jr. and Miller Jr. [11] evaluated the physical properties of PF and polymeric isocyanate resins in the presence of tannin as wood adhesives.
These properties, for instance, their availability and relative ease to chemically modify, make tannins attractive as polymer additives or modifiers. They could be modified by acetylation, chemical hydrolysis, condensation, polymerization, etc. They may also be copolymerized with iso-cyanates, formaldehyde, amino plast or phenolic resins to elaborate thermosetting binders for increasing the compatibility with polyolefin. Finally, for reducing or replacing the side effects of synthetic antioxidants, many studies have focused on the natural antioxidants, especially on forming plant origin materials [12] - [15] . Therefore, by some of chemical modifications, tannins derivatives become more suitable to be used for preparation of adhesives and resins.
The objectives of this study were focused on:
1: Evaluation the cross-linking behavior of PF in the presence of TF;
2: Determining the effects of TF on the kinetic properties of curing reaction of PF;
3: Preparing copolymer structure from Eucalyptus tannin and PF.
2. Materials and Methods
2.1. Isolation of Tannins
Eucalyptus tannins are used after isolation from the outer bark of Eucalyptus trees, the isolation process was carried out by refluxing the bark powder with sodium hydroxide (Fluka) 2% aqueous solution for 24 hrs. Tannins was isolated and then used as sodium salt after filtrations and drying at room temperature.
2.2. Synthesis of Tannin-Formaldehyde Resins
Ten grams from isolated tannin (without further purification) were dissolved in 50ml water. The pH of this solution was adjusted at 10 - 10.5. Then the solution was heated at 80˚C with continues stirring for 75 min. 50 ml of formalin solution (37 - 41)% w/v (H&W) were added to this solution, wise the temperature was set at 60˚C for 3 hr. With continues stirring, the resin solution naturalized with phosphoric acid (Fluka) 10% solution at the end of reaction. Then the water and the unreacted formaldehyde were evaporated under reduced pressure at 60˚C.
2.3. Synthesis of Phenol-Formaldehyde Resin
Phenol-formaldehyde synthesized from phenol (H & W) and formalin solution (formaldehyde) by follow the preparation method and analysis methods which are well described into references [2] and according the reactions and conditions illustrated in the following method:
10 g phenol and 0.5 g NaOH were mixed and dissolved with 50 ml water at room temperature into three neck round. After full dissolving, 25 ml from formalin solution were added slowly during 5minutes with continuous stirring by magnetic starrier. The reaction was carried out at pH 10.5 - 11 and at 60˚C for 3 hrs. At the end of the reaction the pH was adjusted for pH 7 - 6 by phosphoric acid solution 10%v/v. The water and unreacted formaldehyde were evaporated under reduced pressure by using the rotary evaporator.
2.4. Synthesis of Tannin-Formaldehyde/Phenol-Formaldehyde Resins (TF/PF)
TF/PF resins at two percentage (20% and 40%) w/w were prepared by mixing the predetermined amount of TF with suitable weight of PF. The mixing process was continued for 15 min.
2.5. Monitoring Curing Reaction
The curing reaction was studied by determining the percent weight loss that occur in the total weight of resin samples, The sample weight (2 - 5) mg was heated in aluminium pan inside the oven under isothermal condition. The curing of these resins was carried out at four different temperature (160˚C, 180˚C, 200˚C, and 220˚C). The changes in the weight of samples was monitoring as function of time. The apparatus that used for this measurement can be referred in reference [16] .
3. Results and Discussion
The curing reactions of phenol-formaldehyde (PF) resin have been studied extensively by many authors due to technological importance of these products [15] [16] . Curing process and curing performance are quite useful for evaluation the activity of resins to polymerization, and for understanding the conditions and requirements of manufacture and application [2] .
In the present study, the cross-linking reaction of pure PF, pure TF and TF-PF with the mixture of 20% and 40% from TF per PF, were studied by monitoring the weight loss percentage per the weight of sample. This technique is based on the amount of curing reaction products (condensation products), such as H2O and CH2O, that evolved from the resin during curing reaction [16] . Figure 1 showed the curing behavior of PF at various temperatures.
As can be seen in Figure 1, the curing reactions of PF occurred at one step, and the rate of weight loss percentage R is dependent especially on the temperature, where it equal to 1.3 %wt・min-1 for curing at 160˚C, while it rise to about 5.4 %wt・min-1 at 220˚C, This behavior was resulted due to the increase the reactants energies. Figure 2 shows the conversion percentage α (the extent of reaction or of the fraction reacted per total amount of all component that can reacted with each other) [17] reached to 88% per total sample weight after 6 min. when the temperature was 180˚C and 200˚C. It was equal to 90% at 220˚C, but decreased to 52% at 160˚C. This phenomenon may be due to the rapid increasing in viscosity of resin before the depleting of all reactive groups at high temperature. Therefor the full time needed for the curing reactions to reach to the end point were shorter with increasing the temperature.
Generally, the standard integrated expression for first-order reaction (PF curing in this state) is shown in Equation (1) [18] :
Figure 1. Curing curves of PF at various temperatures.
Figure 2. Degree of conversion (α) during PF curing at various temperatures.
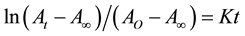 (1)
(1)
where AO, At and A∞ are the initial concentration, concentration at time (t) and final concentration of reactants A, respectively. K is the rate constant of reaction.
Figure 3 was obtained by plotting the left side of Equation (1) versus reaction time at various temperatures, which showed a straight line, indicating the reaction of PF curing follows the first-order reaction. The rate constant at different temperature can be obtained by the slope of this line [18] .
Good linear relations were yield when plotting lnK against 1\T・K−1, as shown in Figure 4, than the activation energy (Ea) for phenol formaldehyde and other resins can be found from Arrhenius relation, Equation (2):
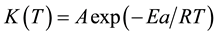 (2)
(2)
where: A is pre-exponential factor, R is the gas constant, Ea the activation energy, and T the absolute temperature/K. Thus the slop of straight line at Figure 4 is equivalent to ?Ea\R. The activation energy Ea of PF thermal curing has values closed to 26.6 kJ・mol−1.
By comparison of the curing behavior of TF resin with that of PF, Figure 1 with Figure 5, there is single curing stage in all temperatures, but the rate and the degree of conversion of curing reaction increas gradually with increase the temperature. Whereas, a great conversion percentage was obtained at 200˚C and 220˚C. The suitable rate of weight loss is about 2.56 %wt・min-1 at 160˚C and 5.58 %wt・min-1 at 200˚C. At 160˚C, the total time for the curing reaction was about 14min., while only several minutes were needed for this reaction when carried at 220˚C.
The chemical reactivity of TF resin to cross-linking is like to that of PF. Although these curves into Figure 5 and Figure 6 indicate that the highest cross-linking density occurs during TF curing reaction. Also at 160˚C the higher levels from curing reactions of TF have been completed during time about 16 min. or less. The curing reaction of TF follow first-order reaction low, Figure 7 shows result, and the activation energy can be obtained from the slope of the straight line of Figure 8, which was about 30.84 kJ・mol−1.
Figure 1 and Figure 5 show that the curing of TF and PF at 5% wt loss start at nearby times but the activation energy of curing TF about 30.84 kJ・mol−1 was more than that of PF, this founding can be attributed to the high molecular weight and stereo hindrance of tannins structure which have hydroxyl groups per phenolic ring more than a simple phenol. The percentage of conversion for TF curing reaction reaches to 60% during 2 min. when this reaction takes place at 220˚C. Whereas it equil to 15% during 2 min. at 160˚C, as illustrated by Figure 6, all these values lower than that of PF, as clearly shown by Figure 2.
However, the partially replacement PF with TF at 20% and 40% w/w leads to produce TF-PF 20% and TF-PF 40% resins. The curing process of these resins can be carried out at the same of PF curing conditions Figures 9-12, show the curing path of this resins at various temperatures. As can be seen in Figure 9 and Figure 13, the curing process consist of single stage at weight loss rate range 1 - 3.3 %wt・min−1 for TFPF 20% and 1- 3.2 %wt・min−1 for TFPF 40% depending on the curing temperature, although the weight loss percentage of the sample after 10 minutes was about 15% - 27.5% per total weight, and 50% - 100% of cure had been achieved.
Figure 3. Relation between the changes in the amount of uncured PF Equation (1) at various temperatures versus curing time.
Figure 4. Variation of lnK of PF curing versus 1\T・K−1, Arrhenius equation.
Figure 5. Curing curves of TF at various temperatures.
Figure 6. Degree of conversion of curing reaction of TF, at various temperatures.
Figure 7. Relation between the changes in the amount of TF versus curing time.
Figure 8. Variation of lnK of TF curing versus 1\T・K−1.
Figure 9. Curing curves of TFPF 20% at various temperatures.
Figure 10. Degree of conversion of curing reaction of TFPF 20% at various temperatures.
Figure 11. Relation between the changes in the amount of TFPF 20% versus curing time.
Figure 12. Variation of ln K of TFPF 20% versus 1\T・K−1, Arrhenius equation.
It is also interested to note that the reducing in the amount of PF in TFPF resins did not give a significant decreasing in the density of cross-linking reactions. A possible explanation for this finding that the TF can act as a source for methylolic groups has the ability to undergo curing reactions. Accordingly, there is no significant decrease in the concentration of condensation products upon completion the cross-linking. Based on the curves into Figure 10 for thermosetting process of TFPF 20% and Figure 14 for that of TFPF 40%, the complete or ultimate conversion occurs after 8 - 12 min. This behavior agree with increasing in the reaction products and hence extent of cure. After this moment, there is decreasing in the rate of curing and conversion cure that is because occur increased in the molecular weight of thermosetting resins into network structure, and then the medium of reaction or the reactants phase become gel or solid thus the chain propagation reaction become hard. And that may be due to decrease in the concentration of unreacted function groups. Additionally the effects of TF on PF thermosetting can be known by the activation energy and curing rate constant, Figure 11 and Figure 15 show that the reaction occur between methylol groups of TF and that of PF follow first-order reaction law, and the rate constants ranges between 0.175 - 0.583 min−1.
The values of activation energy that calculated by Arrhenus relationship for TFPF resins (Figure 12 and Figure 16) showed highly values for 14.3% - 20.75% increasing per that of PF and TF, where it equal to 40.53 kJ・mol−1 for TFPF 20%, and 39.07 kJ・mol−1 for TFPF 40%.
It is well known the present of two components in the some reaction system make the one reactant effects with other, then the reaction pathway and velocity will change. The nature of interaction and inter-forces between tannins and phenol may effect on the cross-linking reactions. As can be seen that there are no mainly change in the reaction order of curing reaction. On the other hand the participation of TF with PF in the formation of polymeric network make the reaction path and curing properties have new and specific properties.
4. Conclusion
The resins that synthesized from TF and PF were thermally cured. According to curing reaction path and the kinetic data of PF, TF, and TFPF, tannins and tannin-formaldehyde resin can be thermally cured. The participation of tannin-formadehyde with PF to the formation thermosetting network was at high performance. The curing reactions density and acceptable conversion degree of TFPF curing were due to the methylol groups into TF resin. The velocity of weight loss during curing TF and TFPF and kinetic parameters of these resins showed that the eucalyptus tannins had high activity to prepare methylolic resins and it is suitable to be partially replacement into PF.
Acknowledgements
The authors are so grateful to Dr. Mohamed A. Jaber, the manager of Polymer Research Center/University of
Figure 13. Curing thermograms of TFPF 40% at various temperatures.
Figure 14. Degree of conversion of curing reaction of TFPF 40% at various temperatures.
Figure 15. Relation between the changes in the amount of TFPF 40% versus curing time.
Figure 16. Variation of lnK of TF/PF 40% versus 1\T・K−1, Arrhenius Equation.
Basrah. Also more thanks to Dr. Salah Shakir Hashem and to Dr. Waeil Abdalsalam Abdelgafor for their support and encouragement to complete our works.
Cite this paper
Hussein AliShnawa,Ibraheem KadumIbraheem,Ashwaq AboudShenta, (2015) Kinetic Study of Curing Phenol-Formaldehyde/Tannin-Formaldehyde Composite Resins. Natural Resources,06,503-513. doi: 10.4236/nr.2015.610048
References
- 1. Keutgen, W.A. (1969) Phenol-Formaldehyde Resins, Phenolic Resins. In: Mark, H.F. and Gaylord, N.G., Eds., Encyclopedia of Polymer Science and Technology, Plastics, Resins, Rubbers, Fibers, Vol. 10, Interscience Publishers, New York, 1-73.
- 2. Adam, G.A. (2001) Chemistry and Technology of Methylolic Resins, Their Derivatives and IPN. National Journal of Chemistry, 1, 131-157.
- 3. Olivares, M., Aceituno, H., Neiman, G., Rivera, E. and Sellers Jr., T. (1995) Lignin-Modified Phenolic Adhesives for Bonding Radiate Pine Plywood. Forest Products Journal, 45, 63-67.
- 4. Sellers Jr., T. (1990) Survey Reveals Use of Lignins as Partial Substitute for Phenol. Panel World, 31, 26-29.
- 5. Sellers Jr., T., Kim, M.G., Miller, G.D., Haupt, R.A. and Strickland, R.C. (1994) Comparison of Strand Boards Made with Phenol-Formaldehyde Resin and Resins Modified with TVA Acid-Hydrolysis Lignin. Forest Products Journal, 44, 63-68.
- 6. Ahmed, M. and Nazli, S. (1993) The Effect of Bivalent Metal Ions of Tannin Formaldehyde Reaction. Journal of the Chemical Society of Pakistan, 15, 21-29.
- 7. Herbert, H.L. (1989) Condensed Tannin in Adhesives: Introduction and Historical Perspectives. In: Hemingway, R.W., Conner, A.H. and Branham, S.J., Eds., Adhesives from Renewable Resources, ACS Symposium Series 385, American Chemical Society, Washington DC, 155-171.
- 8. Saayman, H.M. (1975) Private Communication. Letter to Reichhold Chemicals (USA), 12 May 1975, from Dr. H.M. Saayman, Chief Research Officer, Leather Industries Research Institute, Grahamstown, South Africa.
- 9. Pizzi, A. (1999) Phenolic and Tannin Adhesives for Panel Products. In: Christiansen, A.W. and Pilato, L.A., Eds., Proceedings of International Contributions to Wood Adhesion Research, Forest Products Society, Madison, 13-30.
- 10. Berg, A., Westermeyer, C. and Valenzuela, J. (1999) Radiate Pin Tannin-Based Adhesives. In: Christiansen, A.W. and Pilato, L.A., Eds., Proceedings of International Contributions to Wood Adhesion Research, Forest Products Society, Madison, 122-126.
- 11. Sellers Jr., T. and Miller Jr., G.D. (1997) Tannin-Extended Adhesives for Bonding Strand board Panels. Abstracts of Technical Sessions and Technical Forum Presentations. Annual Meeting, Forest Product Society, Vancouver, 22-26 June 1997, 34.
- 12. Benyahya, S., Aouf, C., Caillol, S., Boutevin, B., Pascault, J.P. and Fulcrand, H. (2014) Functionalized Green Tea Tannins as Phenolic Prepolymers for Bio-Based Epoxy Resins. Industrial Crops and Products, 53, 296-307. http://dx.doi.org/10.1016/j.indcrop.2013.12.045
- 13. Grigsby, W.J., Bridson, J.H., Lomas, C. and Elliot, J.-A. (2013) Esterification of Condensed Tannins and Their Impact on the Properties of Poly(Lactic Acid). Polymers, 5, 344-360. http://dx.doi.org/10.3390/polym5020344
- 14. Ramires, E.C. and Frollini, E. (2012) Tannin Phenolic Resin: Synthesis, Characterization, and Application as Matrix in Bio Based Composites Reinforced with Sisal Fibers. Composites Part B: Engineering, 43, 2851-2860. http://dx.doi.org/10.1016/j.compositesb.2012.04.049
- 15. Yoneda, S. and Nakatsuba, F. (1998) Effects of the Hydroxylation patterns and Degrees of Polymerization of Condensed Tannin on Their Metal-Chelating Capacity. Journal of Wood Chemistry and Technology, 18, 193-205. http://dx.doi.org/10.1080/02773819809349576
- 16. Khalaf, M.N., Shnawa, H.A., Goda, M.K., Lazem, M.A. and Abd-Alemam, D.A. (2008) Phenolic Antioxidant for Polyolefins from Grafted Phenol on Polyethylene Wax. Macromolecular Symposia, 274, 184-188. http://dx.doi.org/10.1002/masy.200851425
- 17. Turi, E.A. (1981) Thermal Characterization of Polymeric Materials. Academic Press, New York, 435-465.
- 18. Chang, R. (2000) Physical Chemistry for the Chemical and Biological Sciences. 3rd Edition, University Science Books, California, 448-471.
NOTES
*Corresponding author.


















