Open Journal of Endocrine and Metabolic Diseases
Vol.3 No.8(2013), Article ID:41149,8 pages DOI:10.4236/ojemd.2013.38040
One-Time Injection of Parathyrin Increases Glucose Tolerance
1Federal State-Financed Establishment of Science, State Scientific Center of Russian Federation, Institute of Biomedical Problems of the Russian Academy of Sciences, Moscow, Russia
2Institute of Physiology Named by I. P. Pavlov of the Russian Academy of Sciences, St.-Petersburg, Russia
Email: butalana07@list.ru
Copyright © 2013 Svetlana Stepanovna Moisa, Alexander Danilovich Nozdrachev. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 25, 2013; revised October 25, 2013; accepted November 2, 2013
Keywords: Parathyrin; Calcium Metabolism; Glucose Metabolism; Calcium Channel Blocker; Insulin Agonist; Glucose Tolerance; Glucoregulating Hormone
ABSTRACT
One-time injection of domestic preparation of bull parathyrin—parathyreoidin (1 U/100g body weight, intramuscular) leads to a significant decrease of the blood glucose level and an increase of the serum calcium level in rats. There is a close negative correlation established between glucose and calcium level (r = −0.813, P > 0.02). The calcium laktat load (9 mg) per os induces the same changes. There is a functional negative correlation established between glucose levels and total calcium content (r = −0.997, P < 0.01). It proves that the decrease of the blood glucose level under parathyrin injection is connected with hypercalcaemia. Parathyrin causes the reduction of the dynamics of hyperglycemia under the glucose load per os (30% solution, 1 ml/100g), i.e. parathyrin increases glucose tolerance. Glucose consumption by muscle (diaphragm) and adipose (epididymal) tissues in vivo and in vitro does not alter under parathyrin and it does not affect the stimulating effect of insulin on the process. The combinative effect of parathyrin and calcium channel blockers—isoptin (5 mg/kg, intraperitoneal) or nifedipin (1 mg/kg, intraperitoneal) reveals more intensive and lasting decrease of the blood glucose level in comparison with only parathyrin effect and more reduction of the dynamics of hyperglycemia under the glucose load. Thus, parathyrin decreases the blood glucose level, increases glucose tolerance and does not effect insulin resistance.
1. Introduction
In previous investigations [1-10], we established that the effect of calcitonin on glucose level reveals hyperglycemia, glucose intolerance and insulin resistance. It was interesting to study the effect of other calcium-regulating hormone—calcitonin antagonist—parathyrin on glucose homeostasis. We consider that there appear to be sufficient reasons for the investigation of parathyrin effect on glucose level, the type of alimentary hyperglycemia and insulin-stimulated glucose consumption by muscle and adipose tissues. Moreover, a special interest was in parathyrin effect against the background of calcium channel blockers treatment. Here we studied in experiments in rats the effect of domestic preparation of bull parathyrin—parathyreoidin on the blood glucose and calcium level, the dynamics of hyperglycemia under the glucose load and glucose consumption by muscle and adipose tissues in vivo and in vitro, and its effect on glucose homeostasis under calcium channel blockers treatment.
2. Method of Investigation
Experiments were performed on 266 male Wistar rats weighing 100 - 150 g. In the 1st series animals received an intramuscular injection of bull parathyrin (“Parathyreoidin”, 1 U/100g of body weight) and the blood glucose concentration and the total content of serum calcium were determined at rest on an empty stomach (initial level) and every 30 minutes after hormone administration over 240 min. Animals of the 2nd series received the calcium laktat load (9 mg) per os and on the analogy of series 1st the blood glucose level and the total calcium content were determined. Blood samples in rats of the 1st and 2nd series were taken under the light ether anesthesia from dissected femoral vein. Rats of the 3rd series received the analogous dose of hormone as in the series 1st and after 30 minutes intraperitoneally nifedipine (1 mg/kg) or isoptin (5 mg/kg), responsibility, and the blood glucose level (30 - 120 min) determined. Animals of the 4th series received the glucose load: 30% glucose solution per os 1 ml/100g body weight and then blood samples were taken every 30 minutes (30 - 240 min) for measuring of glucose concentration. In 5 days the same animals received the analogous injection of hormone and then the glucose load as described above. In the 5th series we studied parathyrin effect on the type of alimentary hyperglycemia against the background of calcium channel blockers treatment. Animals received injection of parathyrin as in the 1st series and 30 minutes later intraperitoneally nifedipine or isoptin similar animals of the 3rd series and then the glucose load was conducted as in the 4th series and the blood glucose level (30 - 120 min) determined. Blood samples in animals of the 3rd, 4th and 5th series were taken from the caudal vein. Experiments in the 6th series parathyrin effect on glucose consumption by muscle (diaphragm) and adipose (epididymal) tissues were conducted in rats in vivo and in vitro. In vivo: parathyrin was administered intramuscularly in a dose of 1 U per 100 g of body weight, 60 min later the rats were decapitated. For stimulation of glucose consumption by muscle and adipose tissues, insulin was injected intramuscurlarly in a dose of 1 U per 100 g of body weight, the animals were sacrificed 60 min later. For evaluation of parathyrin effect on insulin-stimulated glucose consumption by tissues parathyrin was administered 30 min before insulin injection and the rats were decapitated 1 h after insulin administration. Isoptin was administered intraperitoneally in a dose of 5 mg/kg, the animals were sacrificed 60 min later. For the investigation of isoptin effect on combined action of parathyrin and insulin the last was injected 30 min after the combined administration of these preparations. The rats were decapitated 1 h after insulin administration. In vitro: in Krebs-Ringer medium (1 ml) added 0.1 U/ml parathyrin; 0.5 U/ml insulin; 5 mkg/ml isoptin and 6 mkg/ml Bay-K 8644. Under combined effect above indicated preparations were added in the medium simultaneously. The chilled segments of adipose and muscle tissue in 100 - 200 mg were put up in the Krebs-Ringer solution containing 11.1 mmol/l glucose. After 2 h incubation in Varburg’s apparatus performed under 37.7˚C and 60 - 80 rockings in minite. About glucose consumption by tissue were determined on the difference of glucose content in the medium before and after the incubation. Control was glucose consumption by muscle and adipose tissues of intact rats. The blood glucose concentration was measured by the method of Frank-Kirberger [11] and the total calcium content was assayed by complexono-metric method with murexide indicator [12]. The data processed statistically using Student-Fisher tests.
3. Results and Discussion
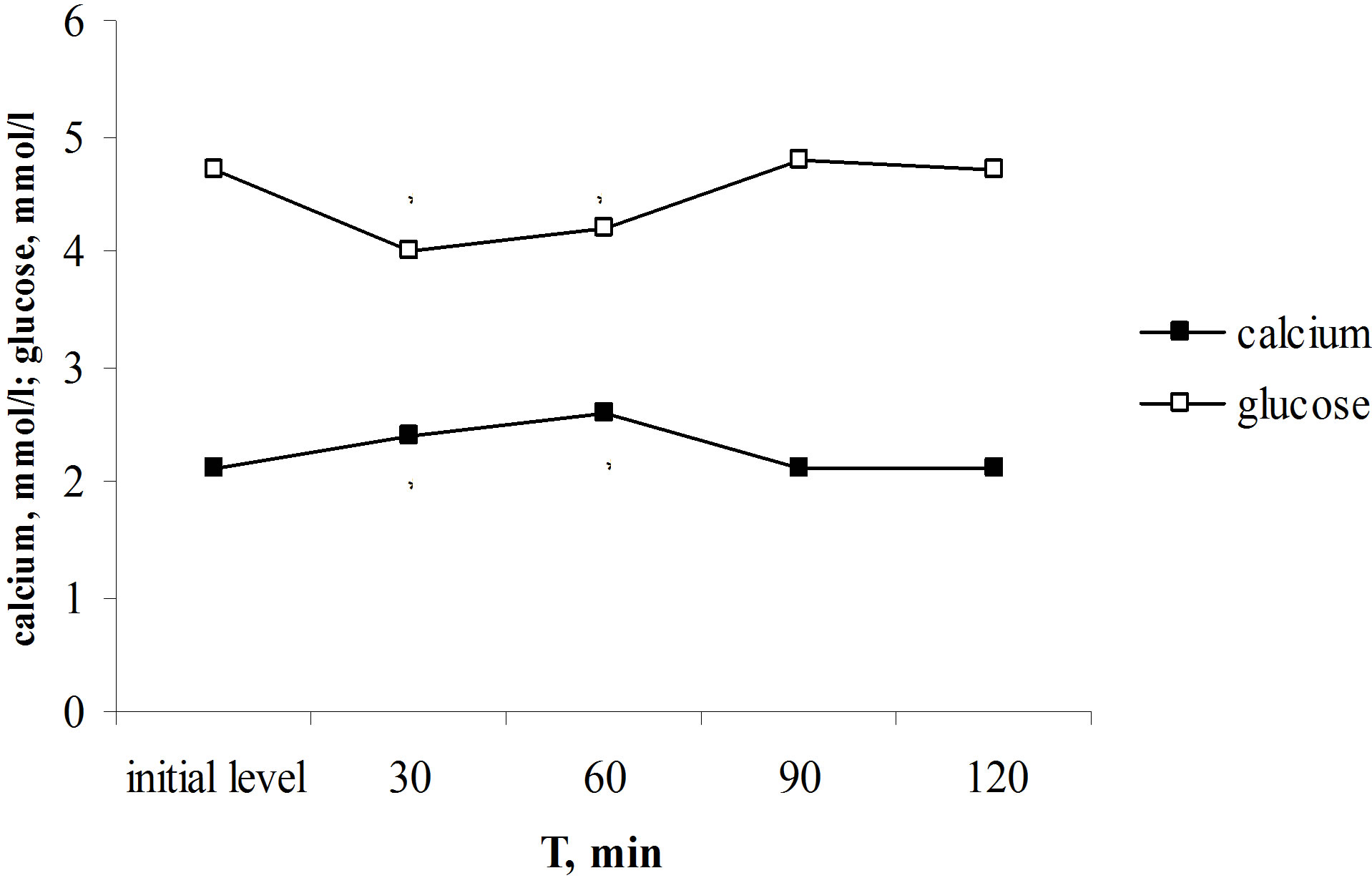
Initial levels of blood glucose and total calcium in rats were normal. Parathyrin injection increased the content of total calcium in blood plasma (from 2.1 ± 0.2 to 2.6 ± 0.03 mmol/l, P < 0.001) and in addition decreased the blood glucose level from 4.7 ± 0.02 to 4.0 ± 0.1 mmol/l (P < 0.001). The normalization of investigating parameters occurred to the 90th min (Figure 1(a)). The decreasing of glycemia induced by parathyrin injection coincides with the increasing of the total calcium level induced by parathyrin. There is a close negative correlation was established between glucose levels and total calcium content (r = −0.813, P < 0.02). Thus, parathyrin administration induced the reliable decreasing of the blood glucose level.
For elucidation of hypercalcaemia role in decreasing of the blood glucose level under parathyrin administration we were carried out the tests with the calcium laktat
 (a)
(a)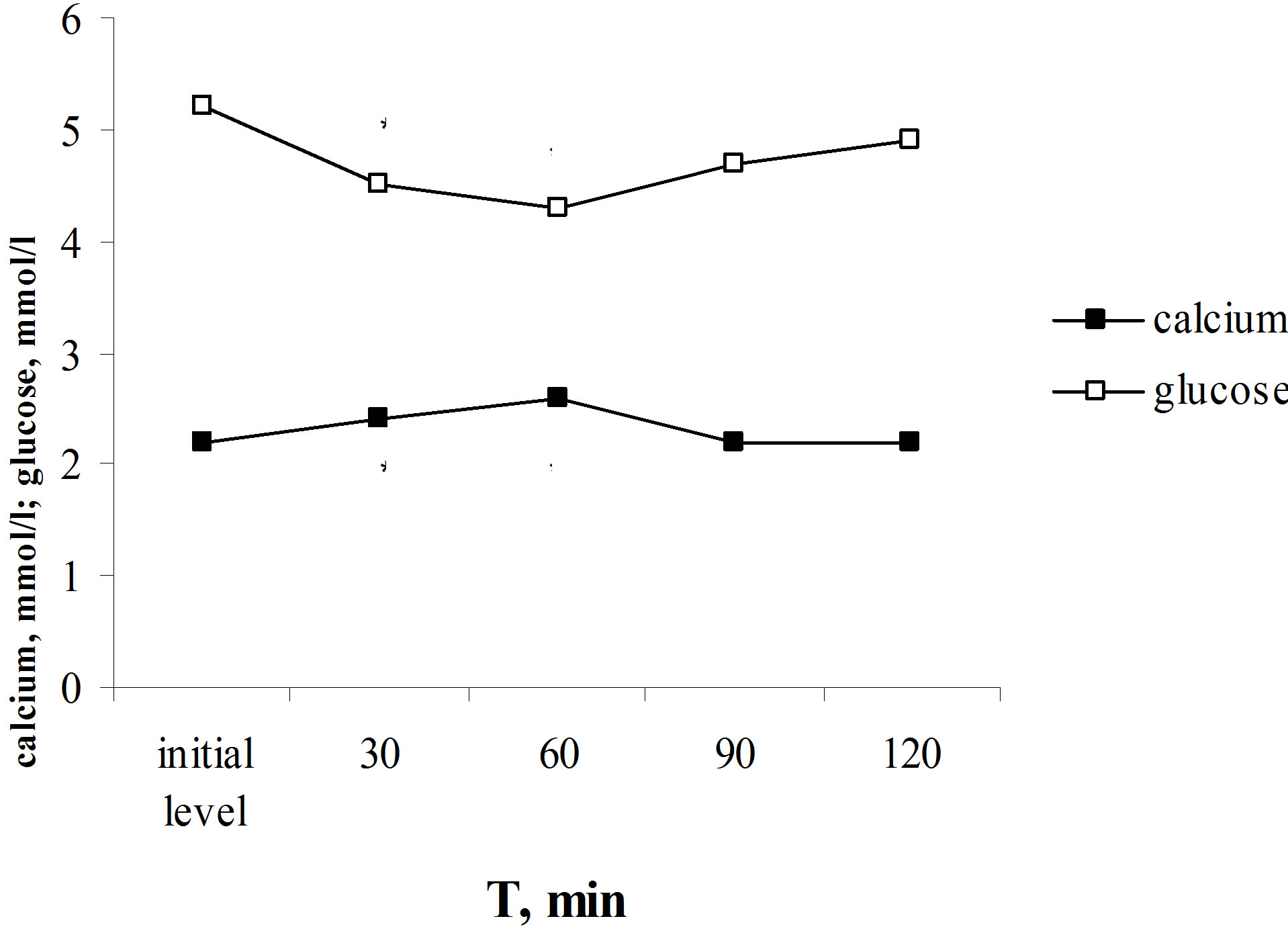 (b)
(b)
Figure 1. Effect of one-time parathyrin injection (a) and the calcium laktat load (b) on glucose level and total calcium content in blood serum in rats. *The reliability of results differences under the initial level.
load. The calcium laktat load provoked the similar alterations of glucose and calcium levels as parathyrin administration—the increasing of calcium level and decreasing of the blood glucose level (Figure 1(b)). Thus, the total calcium content in blood serum increased from 2.2 ± 0.03 to 2.58 ± 0.1 mmol/l, P < 0.001, and glucose level decreased from 5.2 ± 0.01 to 4.3 ± 0.02 mmol/l, P < 0.001. The total calcium content returned to the initial level in 90 min and glucose level—in 120 min after the calcium laktat load. There is a functional negative correlation was established between glucose levels and total calcium content (r = −0.997, P < 0.01).
The findings testify to the effect that the decreasing of glucose level after parathyrin injection caused by hypercalcaemia. In previous series under parathyrin administration the decreasing of glucose level in 30 and 60 min with gradually normalization in 90 min was established in addition the increasing of the total calcium content. It was logically supposed that in the increasing of glucose level to the initial values the endogenous calcitonin takes part, as it is known that calcitonin reveals hyperglycemic effect [2,3,5] and the increasing of the blood calcium content stimulates it secretion. There is a reciprocive interconnection between calcitonin and parathyrin secretion. For confirmation these suppositions we considered it will be reasonable to study parathyrin effect on glucose level against the background of calcium channel blockers —isoptin and nifedipin administration. As it was earlier we established the inhibitory effect of these preparations on calcitonin hyperglycemic effect [7,8], it can suppose that calcium channel blockers administration slows down the restoration of glucose level to the initial value after parathyrin injection. The results of performing experiments are in Figure 2.
Parathyrin injection against the background of isoptin or nifedipin induced more intensive and lasting decreasing of glucose level and the restoration to the initial value didn’t yet occur after 120 min (Figure 2). Thus, calcium channel blockers intensify hypoglycemic effect of parathyrin. Apparently, it can suppose that calcium channel blockers inhibiting hyperglycemic effect of the endogenous calcitonin, stimulated by hypercalcaemia, which induced parathyrin injection, inhibit the restoration of glucose level by calcitonin to the initial value. The findings testify about calcitonin participant in the increasing of glucose level to the initial value after parathyrin injection and once more confirm that calcium channel blockers inhibit calcitonin hyperglycemic effect, in this case the endogenous calcitonin.
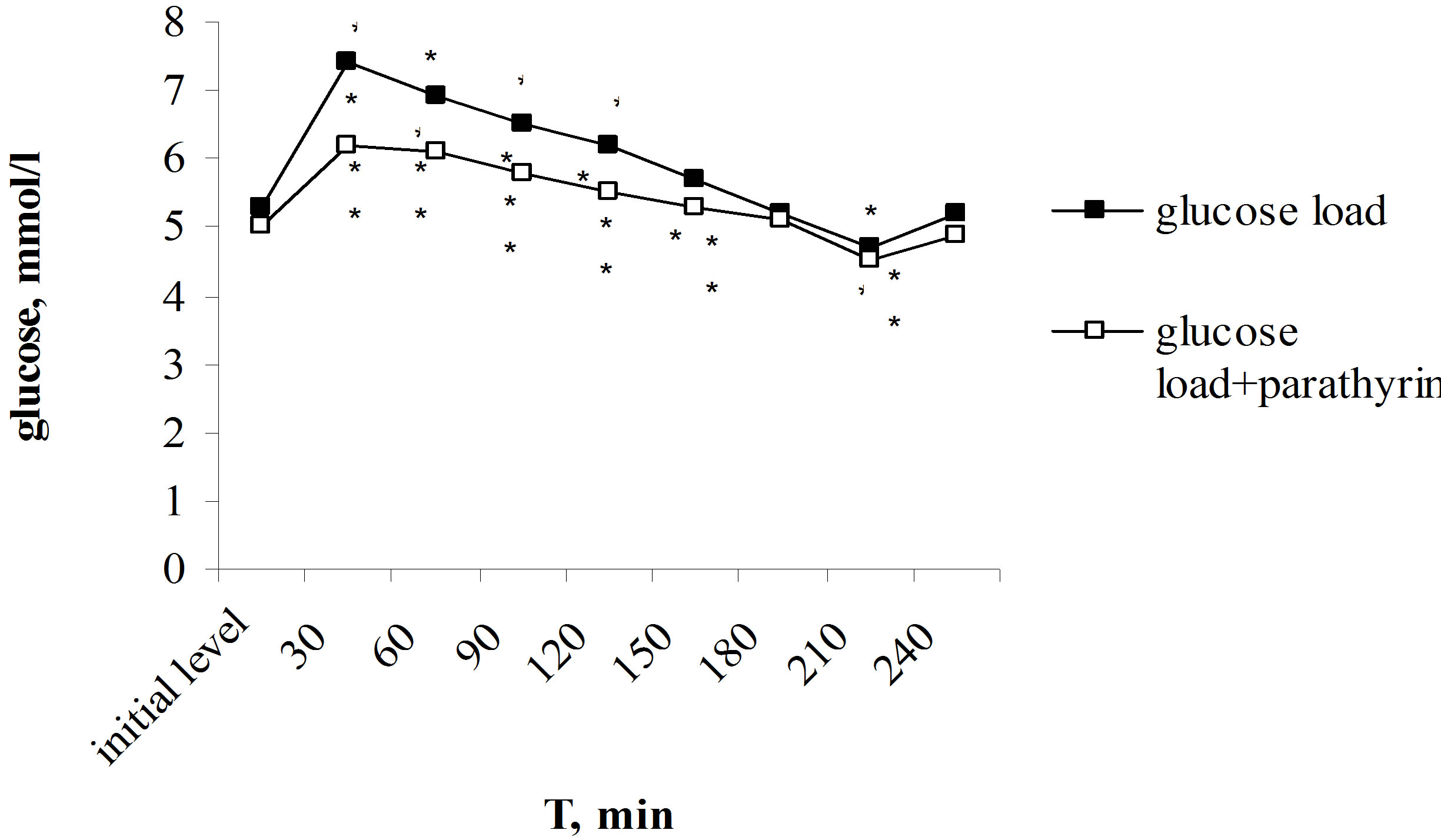
Of particular interest was to study parathyrin effect on the dynamics of hyperglycemia under glucose-tolerance test. Unlike calcitonin, provoking glucose intolerance [3,4,6], parathyrin, on the contrary, decreasing the blood glucose level, reduced the degree of hyperglycemia. Maximal rise of glucose level after the glucose load reached on 30th min and consisted 7.4 ± 0.2 in comparison with 5.3 ± 0.2 mmol/l, P < 0.001 of the initial level. Glucose level was reliable higher the initial level over to 120 min, then the gradually decreasing with the normalization in 180 min occurred, in 210 min it was reliable below the initial level, and in 240 min returned to the initial value again (Figure 3(a)).
Against the background of parathyrin injection the glucose load provoked the reliable hyperglycemia over to 150 min, then normalization of glucose level occurred in 180 min, in 210 min glucose level was below the initial value too and returned to norm in 240 min. However, the expression degree of hyperglycemia was essential lower than under only glucose load. Thus, maximal rise of glucose level also occurred in 30 min but it was reliable lower than under only glucose load—6.2 ± 0.2 in comparison with 7.4 ± 0.2 mmol/l, P2 < 0.001, responsively. Normalization of glucose level also came in 180 min, it reduced lower the initial level in 210 min and returned to norm in 240 min. It should be noted that under parathyrin injection the expression of hyperglycemia was reliable less throughout all over intervals of the investigation (Figure 3(a)). The improving of tolerance to hyperglycemia found its expression in values of hyperglycemic and hypoglycemic coefficients. Thus, hyperglycemic coefficient was reliable lower: 1.24 ± 0.03 in comparison
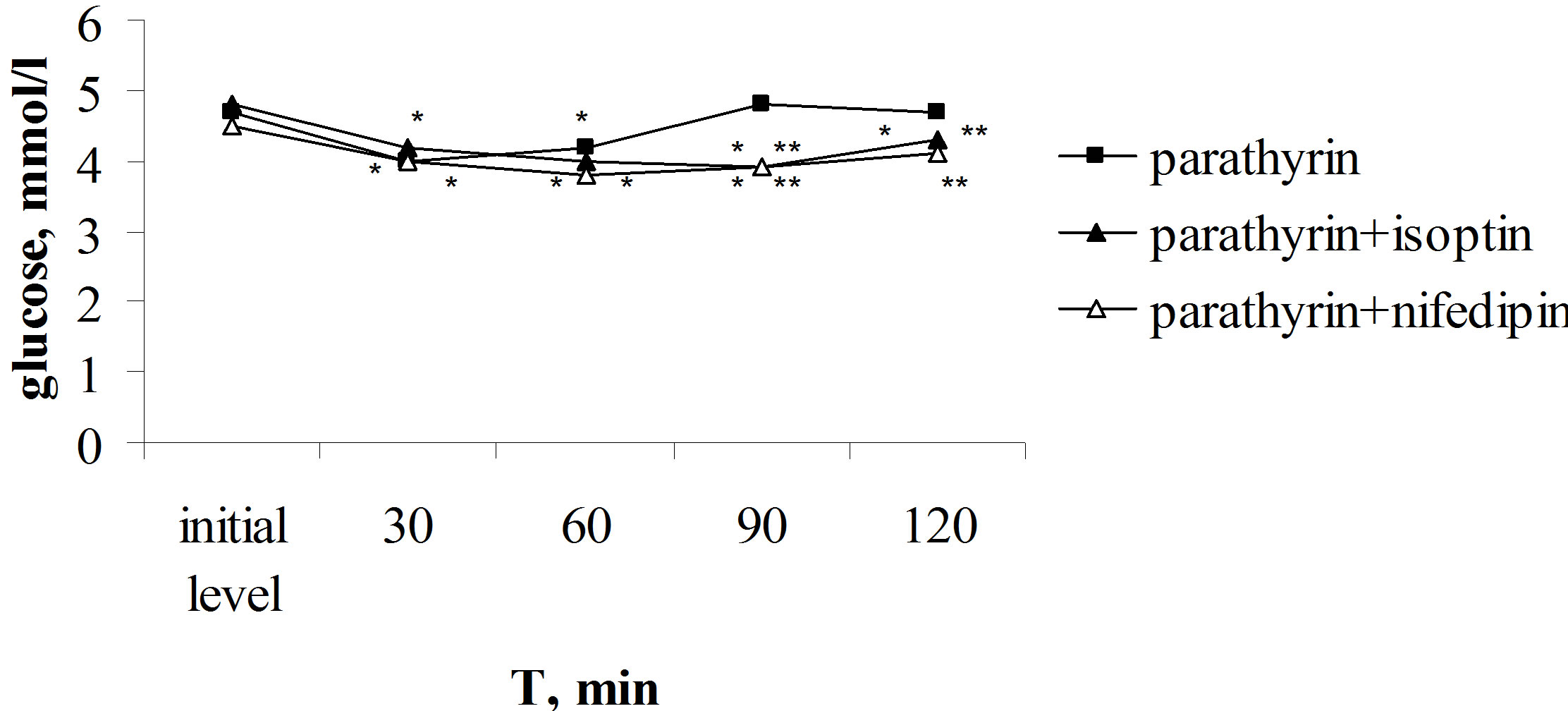
Figure 2. Effect of parathyrin on glucose level against the background of isoptin and nifedipin administration. *The reliability of results differences under the initial level. **The reliability of results differences against the background of parathyrin.
 (a)
(a)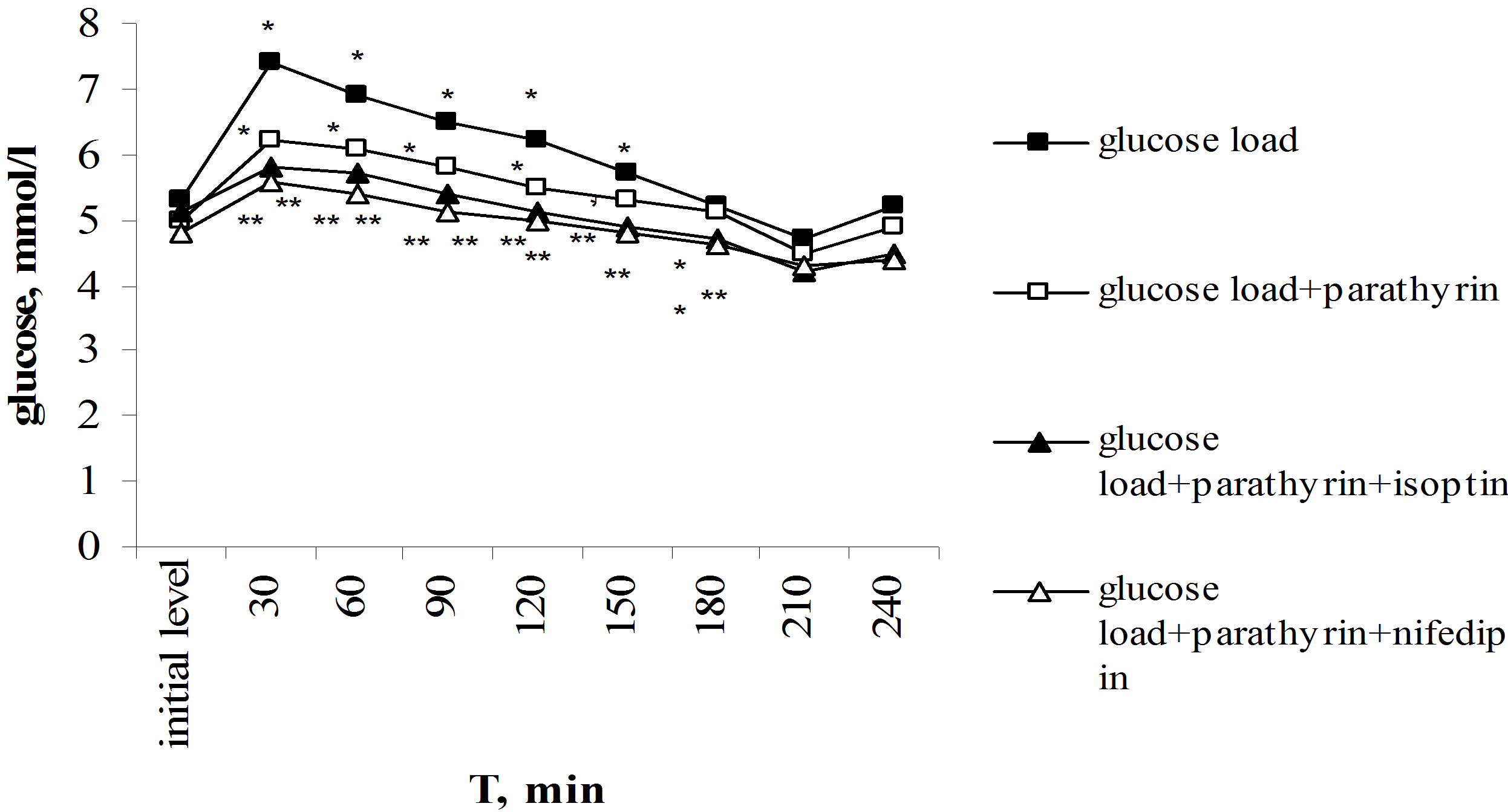 (b)
(b)
Figure 3. Effect of one-time parathyrin injection on the type of alimentary hyperglycemia under control (a) and against the background of calcium channel blockers (b). *The reliability of results differences under the initial level; **The reliability of results differences under the glucose load; ***The reliability of results differences against the background of isoptin and nifedipin.
with 1.396 ± 0.04 against the background of only glucose load (P < 0.001).
Thus, parathyrin injection provoked the decreasing of hyperglycemia induced by the glucose load, i.e. increased glucose tolerance.
Of special particular was to study parathyrin effect on the type of alimentary hyperglycemia against the background of calcium channel blockers. Isoptin or nifedipin administration together with parathyrin under the glucose load led to much more decreasing of hyperglycemia (Figure 3(b)). Thus, against the background of isoptin and parathyrin administration the reliable decreasing of hyperglycemia occurred in comparison with the data under only glucose load. Throughout all over intervals of the investigation (30 - 240 min) glycemia level was reliable lower, the values of glycemic coefficients was also decreased. Normalization of glucose level didn’t yet occur 240 min later after the glucose load, its level stayed yet lower than the initial level. Analogous data were also received under combined nifedipin and parathyrin administration (Figure 3(b)).
So, calcium channel blockers led to much more decreasing of hyperglycemia under glucose-tolerance test against the background of parathyrin administration.
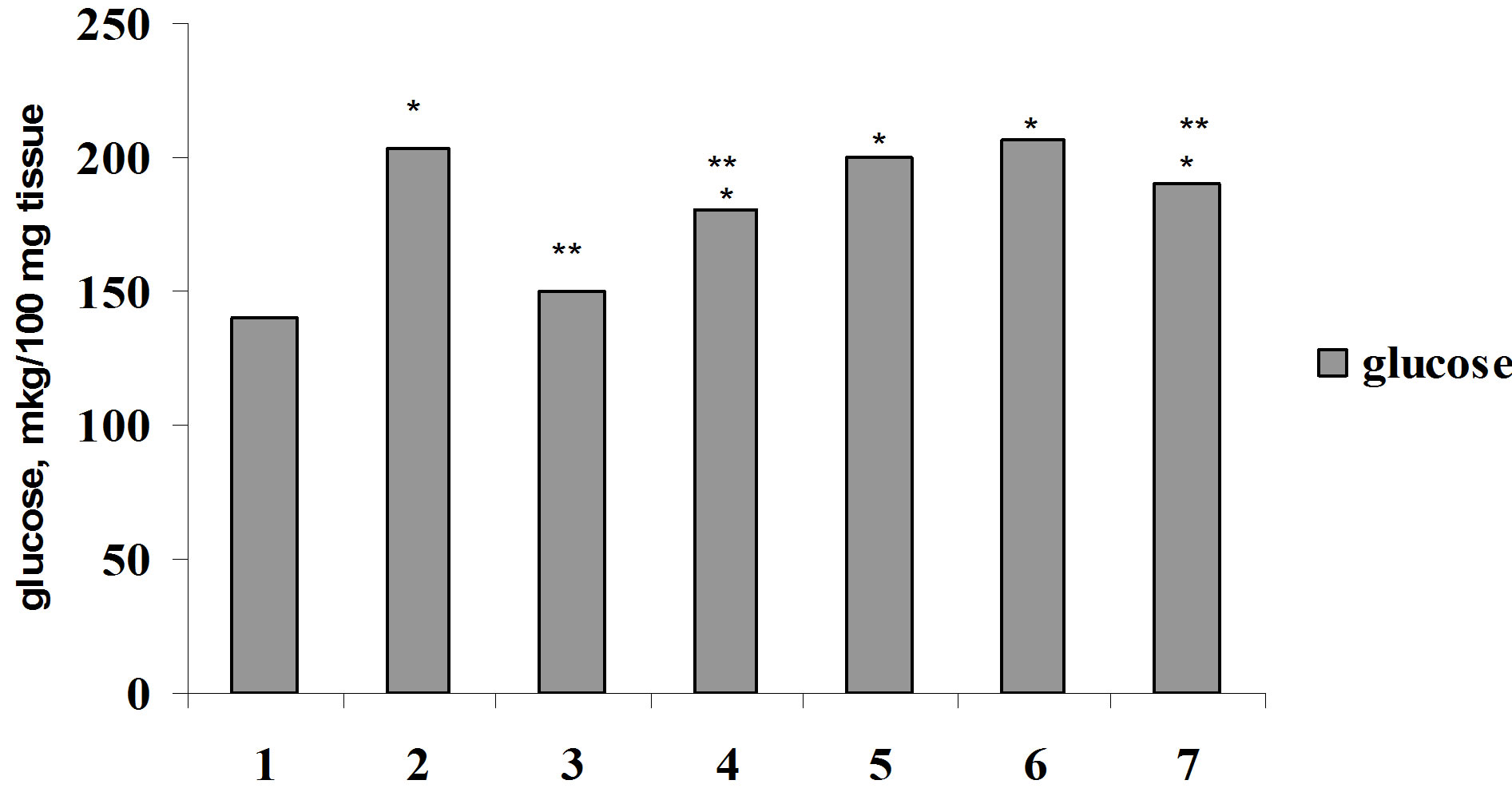
For elucidation of Ca2+ role in biological effect of insulin on glucose consumption by muscle and adipose tissue we were carried out the investigation on the studying of the effect of parathyrin, calcium channel blocker, phenilalkilamin derivative—isoptin and activator of calcium channel, dihydropiridin derivative—BayK 8644 on glucose consumption by muscle and adipose tissue in vivo and in vitro. Parathyrin injection in vivo (Figure 4) didn’t change glucose consumption by diaphragm and adipose tissue in comparison with control (P1 > 0.5). Insulin injection provoked a considerable increasing of glucose consumption by muscle and adipose tissue (P1 < 0.001). Against the background of parathyrin administration insulin—stimulated glucose consumption
 (a)
(a)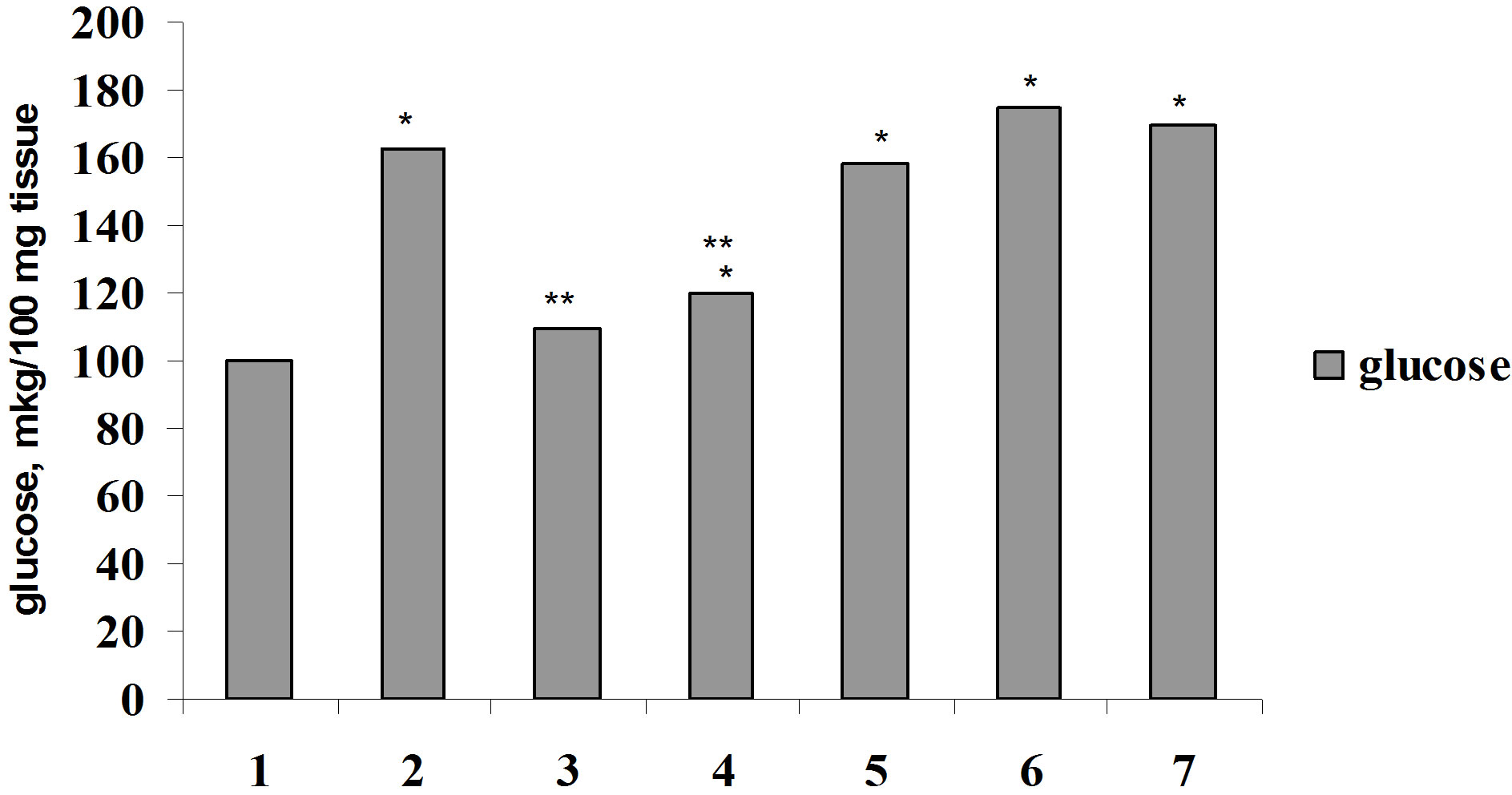 (b)
(b)
Figure 4. Glucose consumption by muscle (a) and adipose (b) tissue under insulin, parathyrin and isoptin in vivo: 1—control; 2—insulin; 3—parathyrin; 4—isoptin; 5—insulin + isoptin; 6—insulin + parathyrin; 7—insulin + parathyrin + isoptin. *The reliability of results differences under control; **The reliability of results differences under insulin effect.
by diaphragm and epididymal didn’t change reliably (P2 > 0.5). Isoptin intensified glucose consumption by muscle and adipose tissue, didn’t change insulin-stimulated glucose consumption and didn’t effect essentially on insulin action under parathyrin administration.
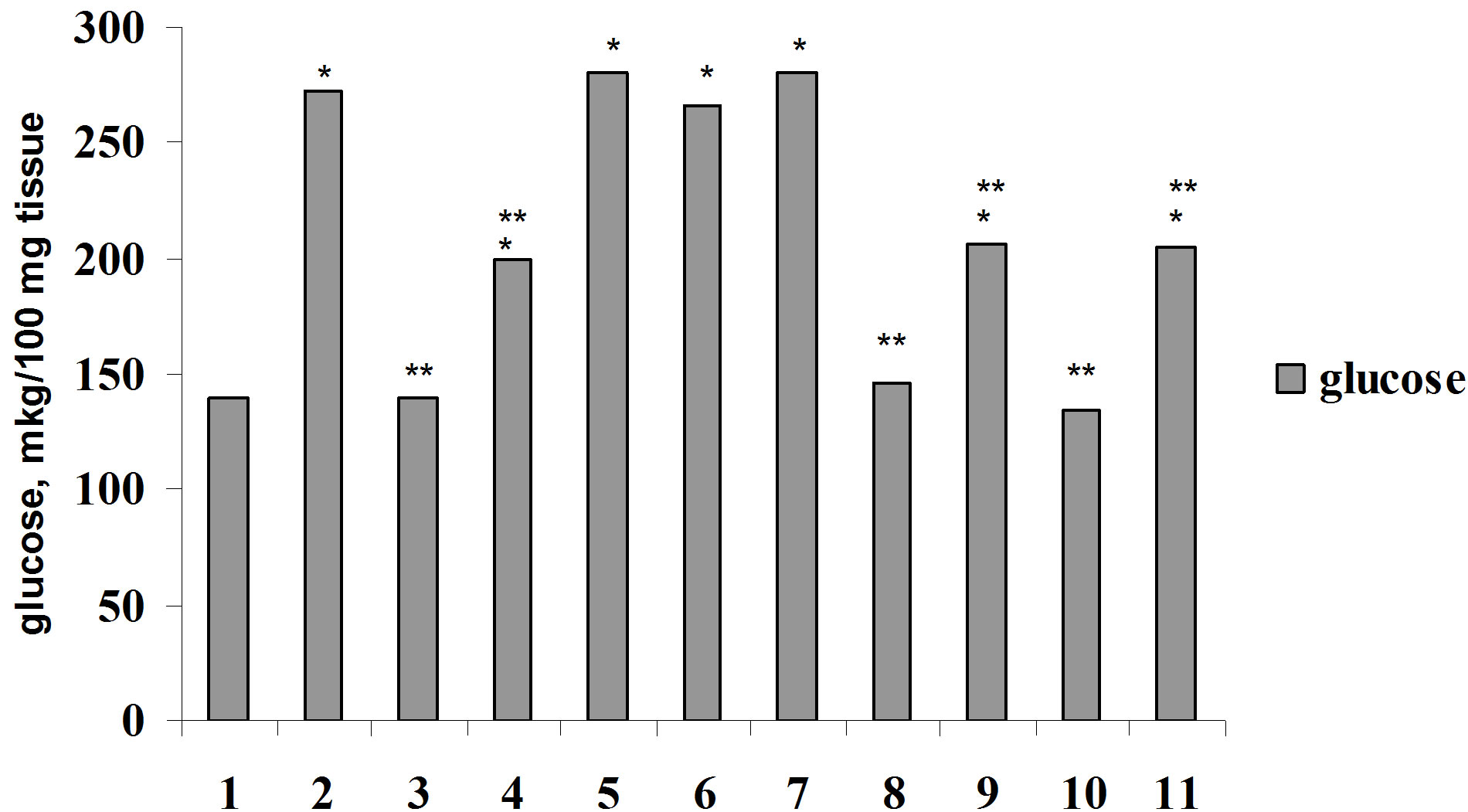
Analogous data were also received in investigations in vitro (Figure 5). The adding in medium Bay-K 8644 didn’t change glucose consumption by diaphragm and adipose tissue in comparison with control (P1 > 0.5). The combined effect of Bay-K 8644 and parathyrin also didn’t change glucose consumption by muscle and adipose tissue. Bay-K 8644, adding in medium, decreased insulin-stimulated glucose consumption by diaphragm and adipose tissue (P2 < 0.05 and P2 < 0.001, respectively). Bay-K 8644 also decreased insulin-stimulated glucose consumption by tissues against the background of parathyrin administration.
Thus, parathyrin didn’t effect on glucose consumption by muscle and adipose tissue, didn’t change insulin-stimulated effect on this process. Regulators of calcium channels—calcium channel antagonist—isoptin didn’t change, but calcium channel activator—Bay-K 8644 decreased insulin effect on glucose consumption by muscle and adipose tissue against the background of parathyrin.
The mechanism of parathyrin effect on glucose level
 (a)
(a)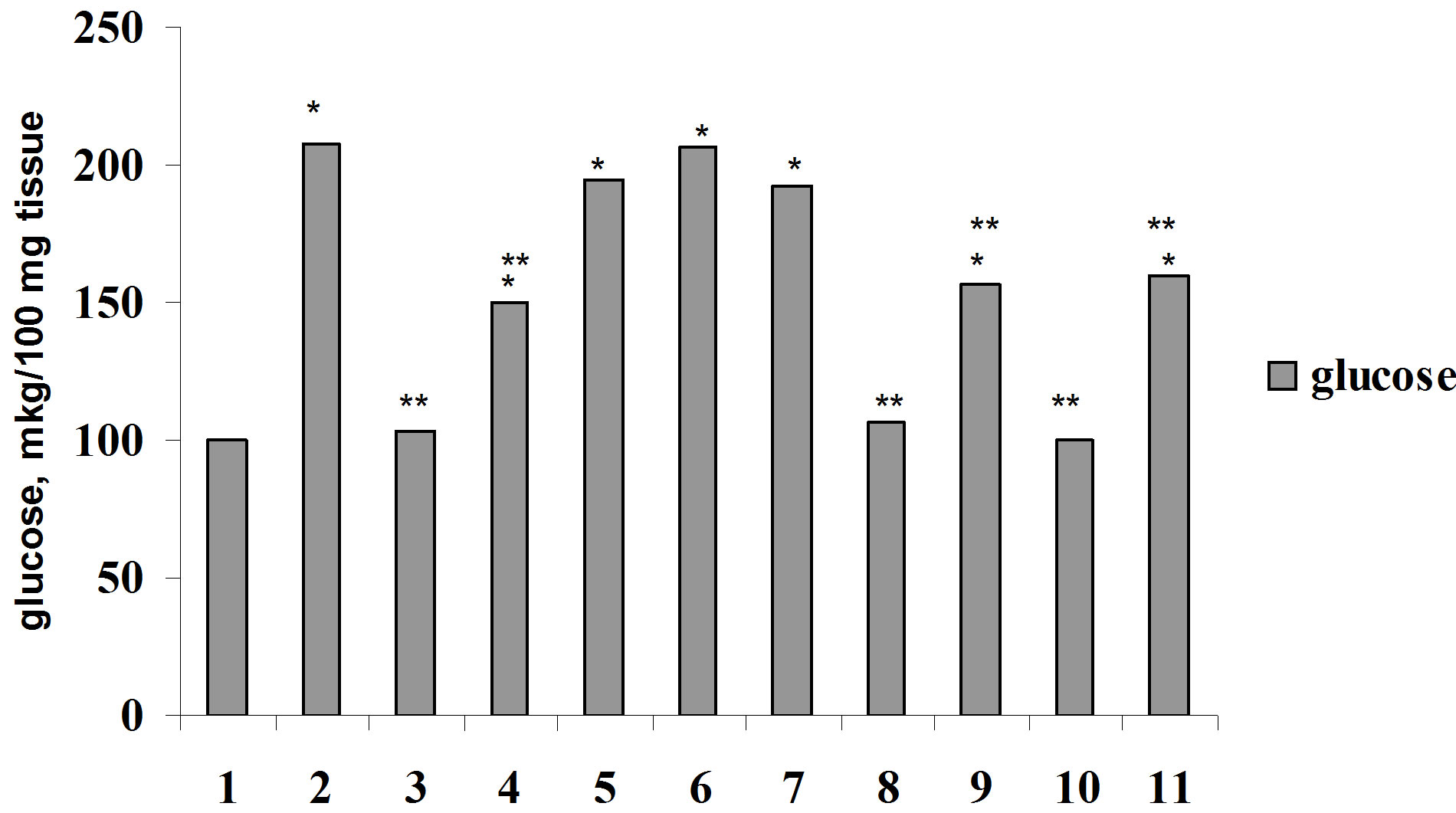 (b)
(b)
Figure 5. Glucose consumption by muscle (a) and adipose (b) tissue under insulin, parathyrin, isoptin and Bay-K 8644 in vitvo: 1—control; 2—insulin; 3—parathyrin; 4—isoptin; 5 —insulin + isoptin; 6—insulin + parathyrin; 7—insulin + parathyrin + isoptin; 8—Bay-K 8644; 9—Bay-K 8644 + insulin; 10—Bay-K 8644 + parathyrin; 11—Bay-K 8644 + parathyrin + insulin. The rest notes as in Figure 4.
remains to be uncertain. Apparently, the decreasing of glucose level under parathyrin administration can be explained by hypercalcaemia induced hormone that it is confirmed in test with the calcium laktat load. In one’s turn, hypercalcaemia leads to the increasing of intracellular Ca2+ concentration in citosol of pancreas ß-cells and to the intensifying of leaving of secret granules, it brings to the increasing of insulin secretion [13], it results in decreasing of the blood glucose level. Besides that, as it is known, Ca2+ level in blood serum above 2.5 mmol/l regulates no one but two hormones—parathyrin and calcitonin [14], and parathyrin doesn’t take part in a rapid regulation of Ca2+ level at that [15], as far as the alterations of calcitonin secretion occur more fast and more brief than the alterations in parathyrin secretion [16].
Therefore, in our case in response to hypercalcaemia in 60 min (parathyrin administration—2.6 mmol/l or the calcium laktat load—2.58 mmol/l) is the result of the increasing of calcitonin secretion by C-cells of thyroid gland. In this connection to 90 min of our investigation either under parathyrin administration or the calcium laktat load normalization of calcium level (hypocalcaemic effect of calcitonin) and glucose level (hyperglycemic effect of calcitonin) was occurred. The participant of calcitonin in the increasing of glucose level to the initial level after parathyrin injection testifies from the experiments with parathyrin administration against the background of calcium channel blockers which were shown much more decreasing of glycemia and yet 120 min after hormone administration glucose level remained lower than the initial level. It confirms once more that calcium channel blockers inhibit hyperglycemic effect of calcitonin [7,8], in this case the endogenous calcitonin, and it testifies of the participant of L-type Ca2+-channels in calcium and glucose homeostasis.
Parathyrin injection led to the reliable decreasing of hyperglycemia and much more it decreasing against the background of calcium channel blockers under glucosetolerance test. It indicates the role of L-type Ca2+-channel in the mechanisms of parathyrin effect on glucose homeostasis and testifies about parathyrin capacity to increase glucose tolerance. Evidently, parathyrin due to hypercalcaemia stimulates insulin secretion which normalizes the blood glucose level and the same one doesn’t make worse glucose tolerance. Analogous data were received under acute hypercalcaemia [17].
A special interest is the data of glucose consumption by muscle and adipose tissue in vivo and in vitro. Just like calcitonin parathyrin didn’t effect on glucose consumption by these tissues but unlike calcitonin [1] it didn’t change insulin-stimulating effect on this process.
The findings allow to make conclusion that parathyrin is calcitonin antagonist not only concerning to the regulation of calcium metabolism, but and glucose metabolism. Parathyrin reliably decreases glucose level, the degree of hyperglycemia under the glucose load, doesn’t effect on insulin-stimulating glucose consumption by muscle and adipose tissue, i.e. increases glucose tolerance.
Establishment of a functional negative correlation between glucose level and the total calcium content indicates about the close interconnection of calcium and glucose metabolism.
With respect to interconnection of calcium-regulating hormones and its effect on glucose and calcium metabolism it can consider that under in vivo the effects of calcitonin and parathyrin can be, in the known degree, the result of changing of circulating Ca2+, in vitro indeed they must consider as the result of direct hormone effect. In other words, in the different cells not having a specialized receptors of calcitonin and parathyrin occur Ca2+-dependent processes subordinated its regulating effects.
It can suppose that parathyrin secretion increases under hypercalcitoninemia and, respectively, hypocalcaemia, that leads to the increasing of the blood calcium level and as a result the increasing of insulin secretion by ß-cells of pancreas. It is established that hypercalcaemia [18] and the increasing of intercellular Ca2+ concentration takes the important role in insulin secretion by ß- cells of pancreas [13]. Calcitonin, in one’s turn, inhibits insulin secretion [19]. In addition, calcitonin increases glucagon secretion, apparently, due to the decreasing of the total calcium content in blood serum, as it is known, that the decreasing of Ca2+ ions concentration leads to the increasing of glucagon leaving from α-cells [20]. Apparently, by this way a reciprocive interrelations between calcitonin and parathyrin secretion and their effect on glucose and calcium metabolism occur, which reveals due to their modulating effect on insulin and glucagon secretion. Therefore, parathyrin acts as insulin agonist and calcitonin—as antagonist. Glucosa, calcium, ß-cells function and calcium-regulating hormones are connected between them by feedback mechanisms [21].
Hypocalcaemia, calcitonin-induced is itself an important factor limiting the entry of Ca2+ in the different tissues and respectively effecting on intercellular disturbance of Ca2+ and Ca2+-dependent processes of cell vital functions. As it is established, Ca2+ increases the sensitivity of ß-cells to glucose [22]. In our view calcitonin decreasing Ca2+ entry to ß-cells provokes the decreasing of its sensitivity to glucose and by this way decreases the number of functioning ß-cells as a result there are insulin resistance and glucose intolerance. It should be noted that the decreasing sensitivity to glucose of secret mechanisms and the decrease of number of functional ß-cells are found in the patients with 2 type diabetes mellitus [23, 24]. A. Mari [25] considers that the decreasing of ß-cells sensitivity to glucose is the dominate defect in glucose intolerance.
Apparently, parathyrin, revealing its effect on nonspecific receptors via Ca2+-dependent processes increases Ca2+-leaving on L-type Ca2+-channels that brings to the decreasing of intercellular Ca2+ concentration in muscle and adipose cells and promotes insulin-stimulated mobilization of GLUT-4 from intercellular store on plasmatic membranes.
Thus, parathyrin doesn’t change insulin effect on glucose consumption by tissues, doesn’t provoke glucose intolerance and insulin-resistance. As it is known, Ca2+- channels are found in skeletal muscle tissue, liver, pancreas, neuro-endocrine tissue, brain, smooth muscle of vertebrates and other tissues [26] and also in man adiposities [27]. At the same time hypercalcaemia brings to the increasing of insulin secretion [13,18]. In this connection the data of the decreasing of glucose level after parathyrin injection (evidently, due to the increasing of insulin secretion) provokes an interest, i.e. hormone reveals the antagonism of action with respect to calcitonin not only on the level of calcium metabolism but on the level of carbohydrate metabolism. Therefore, parathyrin, just like calcitonin, is a gluco-regulating hormone. The interconnection between calcium-regulating hormones, islet apparatus of pancreas and homeostasis of calcium and glucose occurs by feedback mechanisms.
Beyond all doubt, neuro-endocrine mechanisms of their interconnection require the further investigation. However, our findings on this stage testify about the involving of Ca2+-mechanisms.
4. Conclusion
Thus, under the investigation of parathyrin effect on glucose homeostasis, we established the decrease of glucose level after hormone injection, the decrease of hyperglycemic degree under the glucose load, the increase of glucose tolerance. Besides that, it is exposed that parathyrin doesn’t change tissue insulin resistance. Therefore, it can be considered that parathyrin takes part in neuroendocrine regulation of carbohydrate metabolism being calcitonin antagonist. Parathyrin effect on glucose level intensifies against the background of calcium channel blockers, which indicates the role of L-type calcium channel in the mechanisms of hormone effect.
REFERENCES
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Effect of Calcium-Regulating Hormones and Calcium Channel Modulators on Glucose Consumption by Muscle and Adipose Tissues in Vivo and in Vitro,” Bulletin of Experimental Biology and Medicine, Vol. 148, No. 2, 2009, pp. 171-174. http://dx.doi.org/10.1007/s10517-009-0677-x
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Effect of One-Time Injection of Calcitonin Prepations on Glucose and Calcium Level in Rats of Different Age Groups,” Advances of Gerontoljgy, Vol. 23, No. 1, 2010, pp. 93-97.
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Effect of Calcitonin on the Type of Alimentary Hyperglycemia in Rats of Different Age and Sex,” Advances of Gerontology, Vol. 23, No. 2, 2010, pp. 213-220.
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Calcitonin, a Glucoregulating Hormone,” Vestnik Voenno-Medical Academy, Vol. 4, No. 32, 2010, pp. 188-196.
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Mechanisms of Hyperglycemic Effect of Calcitonin,” Bulletin of Experimental Biology and Medicine, Vol. 150, No. 3. 2011, pp. 320-323. http://dx.doi.org/10.1007/s10517-011-1132-3
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Calcitonin—Contra-Insulin Hormone,” Advances in Gerontology, Vol. 23, No. 3, 2010, pp. 364-370.
- S. S. Butakova (Moisa), “Effect of Calcitonin and Calcium Channel Blockers on Glucose Metabolism,” Materials of All-Russian Conf. with International Participant Devoted to the 85th Anniversary of Foundation of Institute of Physiology Named by I.P. Pavlov of The Russian Academy of Scienses, Sanct-Petersburg, 2010, p. 40.
- S. S. Butakova (Moisa) and A. D. Nozdrachev, “Calcium Channel Blockers Inhibit the Hyperglycemic Effect of Calcitonin,” Bulletin of Experimental Biology and Medicine, Vol. 152, No. 5, 2012, pp. 553-559. http://dx.doi.org/10.1007/s10517-012-1573-3
- S. S. Moisa and A. D. Nozdrachev, “One-Time Injection of Calcitonin Induces Glucose Intolerance in Children with the 1st Degree Obesity,” Journal “Health” USA, Vol. 5, No. 6, 2013, pp. 9-13. http://dx.doi.org/10.4236/health.2013.56A1002
- S. S. Moisa, “Calcitonin Contra-Insulin Action on Glucose Metabolism,” Bulletin of Experimental Biology and Medicine, Vol. 156, No. 8, 2013, pp. 183-185.
- H. Frank and E. Kirberger, “Eine Kolorimetrische Methode zur Bestimmung der ‘Wahren Glucose’ und Galactose in 0.05 rm3,” Blut. Biochem. Ztschr, Vol. 320, 1950, pp. 359-367.
- I. I. Selochnik, A. I. Briskin and E. E. Antonova, “Photoelectrocolorimetric Assay of Calcium Concentration in Serum by the Help of EDTA and Murexide,” Chemical Pharmacological Journal, Vol. 12, No. 10, 1978, pp. 138-140.
- H. S. Kim, S. Yumham, H.-Y. Lee, et al., “C-Terminal Part of AgRP Stimulates Insulin Secretion through Calcium Release in Pancreatic ß RinSmf Cells,” Neuropeptides, Vol. 39, No. 4, 2005, pp. 385-393.
- J. F. Habener, “Pathogenesis of Renal Osteodystrophy— A Role for Calcitonin,” Annual Review of Physiology, Vol. 43, 1981, pp. 211-223. http://dx.doi.org/10.1146/annurev.ph.43.030181.001235
- W. Wang, E. Lewin and K. Olgaard, “Parathyroid Hormone Is Not a Key Hormone in the Rapid Minute-to-Minute Regulation of Plasma Ca2+ Homeostasis in Rats,” European Journal of Clinical Investigation, Vol. 29, No. 4, 1999, pp. 309-320.
- L. J. Deftos, E. G. Watts, D. H. Copp and J. T. Potts, “A Radioimmunoassay for Salmon Calcitonin,” Endocrinology, Vol. 94, No. 22, 1974, pp. 155-160.
- O. Gedik, S. Akalin and Z. Koray, “Effect of Acute Hypercalcaemia on Glucose Tolerance and Insulin Release in Human Beings,” Acta Endocrinologica, Vol. 94, No. 2, 1980, pp. 196-200.
- J. G. Parthemore and L. J. Deftos, “Calcitonin Secretion in Normal Human Subjects,” The Journal of Clinical Endocrinology & Metabolism, Vol. 47, No. 1, 1978, pp. 184-188. http://dx.doi.org/10.1210/jcem-47-1-184
- S. S. Butakova (Moisa), “Calcitonin, a Modulater of Pancreas Secret Process,” Thesis of Addresses, Institute of Physiology named by I.P. Pavlov of The Russian Academy of Scienses, Sanct-Petersburg, 2008, pp. 26-27.
- E. Panzig, W. Besch and K.-D. Rosenbaum, “The Effect of Potassium, Calcium and Magnesium Concentration on Insulin and Glucagon Secretion of the Perfused Dog Pancreas,” Experimental and Clinical Endocrinology & Diabetes, Vol. 86, No. 1, 1985, pp. 61-68.
- G. K. Zoloev, V. D. Slepushkin, R. A. Yakovleva, et al., “Relationships between the Two-Valency Cationes, the Function of the Insular Apparatus of Pancreas and Calcium-Regulating Hormones under the Alteration of Blood Glucose Level,” Human Physiology, Vol. 10, No. 3, 1984, pp. 450-453.
- A. M. Davalli, E. Biancardi, A. Pollo, et al., “Dihydropyridine—Sensitive and—Insensitive Voltage-Operated Calcium Channels Participate in the Control of GlucoseInduced Insulin Release from Human Pancreatic ß-Cells,” Journal of Endocrinology, Vol. 150, No. 2, 1996, pp. 195-203.
- A. B. Jenkins and L. V. Campbell, “Insulin Secretion and Impaired Glucose Tolerance,” Diabetologia, Vol. 53, No. 10, 2010, pp. 2266-2268. http://dx.doi.org/10.1007/s00125-010-1801-1
- B. L. Wajchenburg, “B-cell Failure in Diabetes and Preservation by Clinical Treatment,” Endocrine Reviews, Vol. 28, No. 2, 2007, pp. 187-218. http://dx.doi.org/10.1210/10.1210/er.2006-0038
- A. Mari, A. Tura and A. Natali, “Impared Beta Cell Glucose Sensitivity Rather than Inadequate Compensation for Insulin Resistance Is the Dominant Defect in Glucose Intolerance,” Diabetologia, Vol. 53, No. 4, 2010, pp. 749- 756. http://dx.doi.org/10.1007/s00125-009-1647-6
- E. A. Ertel, K. P. Campbell, M. M. Harpold et al., “Nomencrature of Voltage-Gate Calcium Channels,” Neuron, Vol. 25, No. 3, 2000, pp. 533-535. http://dx.doi.org/10.1016/S0896-6273(00)81057-0
- W. O. Wilkinson, M. B. Zemel and N. Moustaid-Moussa, “Modulation of the Sulfonylurea Receptor and Calcium in Adipocyties for Treatment of Obesity/Diabetes: Patent 6569633 USA, МПК 7 GOIN 33/566, GOIN 33/567,” Artesel Science Inc., 2003. No. 09/592421.

