Open Journal of Pathology
Vol. 3 No. 3 (2013) , Article ID: 33759 , 6 pages DOI:10.4236/ojpathology.2013.33019
Assessment of Diagnostic Accuracy of Bronchoalveolar Lavage Cytology in the Diagnosis of Lung Tumors and Contribution to the Classification of Non-Small Cell Lung Cancer Entities: A Retrospective Clinocopathological Study*
![]()
1Institute of Pathology, University of Rostock, Rostock, Germany; 2Department of Respiratory Medicine, University of Rostock, Rostock, Germany; 3Institute for Biostatistics and Informatics in Medicine and Ageing Research, University of Rostock, Rostock, Germany.
Email: #annette.zimpfer@med.uni-rostock.de
Copyright © 2013 Annette Zimpfer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 5th, 2013; revised May 25th, 2013; accepted June 20th, 2013
Keywords: Lavage Cytology; Accuracy; Lung Cancer; NSCLC; Classification
ABSTRACT
Background: Due to new therapeutic options in thoracic oncology, the pathological diagnosis of bronchial carcinoma has become more challenging. The majority of bronchial cancer is diagnosed from small biopsy specimens and the diagnosis often based on cytological methods. Aims: In this study, we reevaluated cytologic specimens in order to determine the diagnostic reliability of pulmonary cytopathologic techniques performed in our department. Material and methods: In our center bronchial lavage/bronchoalveolar lavage (BL/BAL) specimens are obtained both before and after forceps biopsy (FB) and subsequently processed. Retrospective data from a period of 60 months were retrieved from the institutional files. Sensitivity, specificity, as well as accuracy of cytological tumor typing were determined using histopathology of FB as gold standard. Also, the diagnostic yield of BL/BAL before and after FB was determined. Results: 678 cases were retrieved from the institutional files. The sensitivity and specificity of cytology were 83.0% and 83.4%, respectively. By FB in 3.9% of cytologically diagnosed non-small cell lung carcinomas (NSCLC) a histological assignment to a NSCLC entity was not possible. Conclusions: Cytology is a reliable diagnostic tool in the diagnosis of lung malignancies. High diagnostic accuracy is achieved by a combination of BL/BAL before and after FB. The diagnostic yield of BL/BAL after FB was significantly higher than BL/BAL before FB. Subsequent tumor typing of cytologically diagnosed NSCLC was feasible in more than 95% of cases.
1. Introduction
Lung cancer is the most common cancer in the world and one of the leading causes of death due to cancer in both men and women [1]. Nearly all primary malignant neoplasms of the lung are of epithelial origin. The majority of them are diagnosed as small cell lung cancer (SCLC), adenocarcinoma (AC), squamous cell carcinoma (SCC) and large cell carcinoma (LC). The minority of cases consist of carcinoid tumors [1]. According to recent data the most common subtype of lung carcinoma is AC [2].
Recently, new studies have focused on the problem of diagnosing and subtyping non-small cell lung carcinoma (NSCLC) in cytologyand particularly in small biopsy specimens, and have highlighted the problem of providing a new classification system of adenocarcinomas [3,4]. About 70% of lung cancers are diagnosed in small biopsies or cytologic samples [4]. Especially in patients with advanced-stage disease the subtyping of NSCLC in small biopsies or cytologic samples is of increasing importance due to new therapeutic options and strategies [4-7]. Diagnostic exclusion of SCC assists in identifying patients who may benefit from pemetrexed or bevacizumab therapy [7,8]. Particularly AC histology is a strong predictor for a better outcome with pemetrexed therapy as compared to SCC [4,9]. AC and NSCLC not otherwise specified (NOS) should be tested for epidermal growth factor receptor (EGFR) mutations to predict response to EGFR tyrosine kinase inhibitor therapy [5,6].
Difficulties in subtyping NSCLC in small biopsies or cytologic samples arise especially in poorly differentiated SCC and AC, whereas well to moderately-differentiated SCC and AC are more easily identified. Thus, 10% - 30% of diagnoses in small biopsies and cytology samples are NSCLC, NOS [7,10,11].
Nowadays, fiberoptic bronchoscopy is an excellent tool utilized for the diagnosis and staging of lung tumors, and the recovery of cells and tissues by transbronchial needle aspiration, bronchial/bronchoalveolar lavage (BL/ BAL), bronchial brushing or endobronchial/transbronchial (forceps) biopsy [Schreiber]. Often, BL/BAL, brushings, and forceps biopsies (FB) are combined to increase the sensitivity and a cytohistological diagnosis can be obtained in most cases [12,13]. The usefulness of obtaining washings is still a matter of controversy because the reported sensitivities are quite low [12,14].
The aim of our retrospective study was to determine the diagnostic reliability of lavage cytopathology performed in our department. The question was whether the combined cytohistological analysis done in our institution would help to reduce the percentage of NSCLC, NOS diagnoses to less than 10%.
2. Material and Methods
Retrospective bronchoscopy data from a period of 60 months (from 01/01/2007 to 12/31/2011) of a total of 678 diagnostic cases were retrieved from the institutional files and included in the analyses: These consisted of either combined (i.e. pre-and post FB) bronchial lavage/ bronchoalveolar lavage (BL/BAL) and forceps biopsy (FB) specimens, or single BL/BAL pre or post FB. Only clinically suspicious cases were included in this study. Histological diagnosis of FB was additionally compared with subsequent histological diagnosis, especially with results of computer-tomography (CT-) guided trans-thoracic fine-needle biopsies (TT-FNB) mostly in peripheral lesions.
BL/BAL specimens were obtained by instilling isotonic saline into the conspicuous segment and reaspirating. In 509 cases a biopsy was obtained between two washing/lavage procedures. In 169 cases only one BL or BAL procedure was performed either pre or post FB. All BL/BAL samples were sent to the cytopathology laboratory with clinical and bronchoscopic information. Lavage specimens were centrifuged (2500 rpm, 10 min). Two to 4 slides were prepared from the cell concentrate and stained according to Papanicolaou (Merck). The BAL specimens were sedimented each with 1 ml to 2 to 4 slides and stained with May-Grunwald-Giemsa. Additionally Grocottand Ziehl-Neelsen-stains were only prepared when clinically specific questions were stated. The slides were evaluated by different pathologists separately from the biopsy material. Simultaneously obtained biopsy (FB) material was sent in formalin (4%), and a set of histopathology slides were prepared for histological and immunohistochemical examination. The tumors were classified according to the World Health Organisation’s classification [1]. For cytology, cases with unequivocal malignant features and cases with suspected or atypical cells (“require further evaluation”) were considered to be positive. Slightly modified Bethesda diagnostic categories were adopted for cytological classification (nondiagnostic or unsatisfactory, benign, atypia of unknown significance or suspicious, suspicious for malignancy and malignant cells). Sensitivity, specificity, positive predictive value and negative predictive value of bronchial cytology were determined using histopathology of FB as gold standard. Also, performance data of lavage cytology and FB using final histopathological diagnosis as gold standard was determined. The diagnostic yield of positive cytology cases before and after FB was determined. False positive cases were reviewed. Additionally, the concordance between cytological and histological tumor classification was assessed.
Statistical Analysis
All data were analyzed using the Statistical Program for the Social Science version 17.0 (SPSS Inc., Chicago, Illinois). Computed statistics included medians and ranges for continuous variables, and frequencies and percentage frequencies for categorical variables. Sensitivity, specificity, positive predictive value and negative predictive value of bronchial cytology were calculated according to the literature [15]. To calculate the statistical significance of the diagnostic yields between BL/BAL before FB and BL/BAL after FB the χ2-Test or Fisher’s exact test was performed. All p values resulted from two-sided statistical tests and values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Patient Data
A total of 678 diagnostic cases from 619 patients were included in this study. 420 (62%) patients were male (range 16 - 89 years, arithmetic mean 66.2 years) and 199 (28%) patients were female (range 27 - 89 years, arithmetic mean 63.5 years). According to the endoscopist’s reports, in 204/678 (30.1%) cases an endobronchial tumor was visible. 252/678 (37.2%) and 106/678
(15.6%) of cases could be assigned to a central or peripheral lesion, respectively (see Table 1).
3.2. Statistical Evaluation of BL/BAL Cytology and FB
Diagnosis of malignancy or suspected cancer via BL/ BAL plus FB was established in 413/678 cases (61.9%). By histology, the majority of tumors consisted of 76 SCLC, 125 SCC and 70 AC (see Figures 1(A) and (B)). 14, 30 and 18 cases were classified as NSCLC, LC or sarcomatoid/pleomorphic carcinoma, and “other” tumors (i.e. rare lung tumor entities or metastases, see Figures 1(C) and (D)). 27 cases were classified as “suspicious for malignant cells” or “dysplasia” or “carcinoma in situ”.
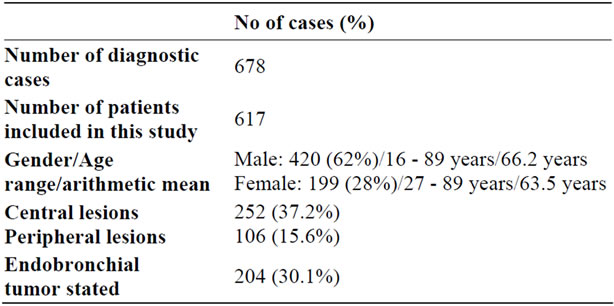
Table 1. Basic patients and endoscopic data.
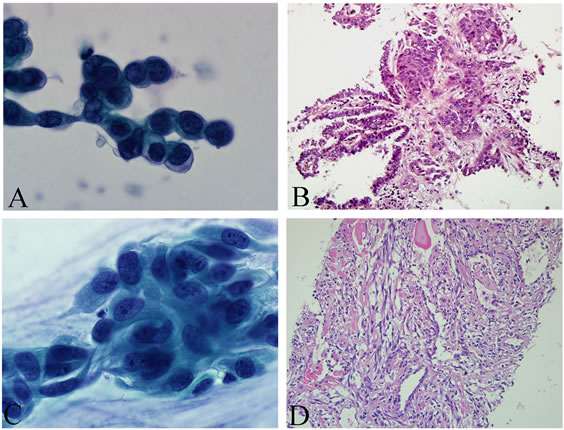
Figure 1. (A), (B) Lavage cytology after peripheral FB (B1 and 2c) of the lung revealed single atypical adenoid cell formations, consistent with cells of an adenocarcinoma (Papanicolaou, 100×, (A)); By FB tumor infiltrations of a solid high grade adenocarcinoma with superficially lepidic adenocarcinoma formations (adenocarcinoma in situ) was found and immunohistochemically confirmed (H&E, 20×, (B)); (C), (D) Lavage cytology revealed atypical epitheloid and spindle cells (Papanicolaou, 100×, (C)); Forceps biopsy material contained no tumor cells. In consecutive performed CT-guided TT-FNB lung infiltration of a malignant spindle cell tumor was seen. After additional immunohistochemical studies a malignant solitary fibrous tumor was diagnosed (H&E, 20×, (D)).
By comparison with FB, sensitivity and specifity of lavage cytology were calculated to be 83.0% and 83.4%, respectively. The positive and negative predictive values were 85.6% and 80.9%, respectively. There were 51/678 (7.5%) false positive results. 29 (56.9%) of these 51 false positive cases were cytologically classified as “suspicious, require further evaluation”. In 22/51 (43.1%) false positive cases a peripheral mass lesion was stated clinically. After evaluation of these 51 cases using additionally histopathological data in 44/51 cases a malignant diagnosis was established. In the remaining 7 false positive cases, 3 inflammatory lesions (one Wegner’s granulomatosis, one chronic organizing pneumonia and one granulomatous bronchitis) had been cytologically misjudged as malignant lesions. In the remaining 4 cases, no further diagnostic work-up was done.
By comparison with final histological diagnosis (after integrating histological diagnosis from CT-guided TTFNB-material, available surgical material, biopsies from metastatic sites or autopsy material), sensitivity and specifity of lavage cytology plus FB were calculated to be 88.7% and 96.5%, respectively. The positive and negative predictive values were 98.3% and 81.1%, respectively.
3.3. Diagnostic Yield of BL/BAL before and after FB
The diagnostic yield of BL/BAL before and after FB was 277/509 (54.4%) and 310/509 (60.9%), respectively (p = 0.023).
3.4. Assessment of Accuracy of Cytologic Tumor Typing
The diagnostic accuracy of cytologic tumor classification varied with cell type and location of tumor. Cytologic typing of SCLC, SCC and AC was confirmed by histology in 97.9% (47/48), 95.6% (86/90) and 90.2% (55/61), respectively (see Table 2).
In 54/678 (8.0%) cases FB was negative. In 23/54 (42.6%) of these cases a radiological peripheral lesion was stated. Further diagnostic work-up by means of CTguided TT-FNB showed in 13 cases a benign result and in 67 cases a malignant diagnosis. In 61/678 (9.0%) cases only histology was positive whereas cytology was negative.
60/678 cases (8.8%) were cytologically diagnosed as NSCLC. By histology, these cases were subclassified as SCC (22 cases), AC (11 cases), LC (19 cases), suspicious cases (5 cases) and 1 SCLC (see Table 2). In relation to the 446 histologically malignant diagnoses the frequency of NSCLC was 0.9% (4/446). Related to 360 malignant und suspicious diagnoses in FB 14 (3.9%) NSCLC could
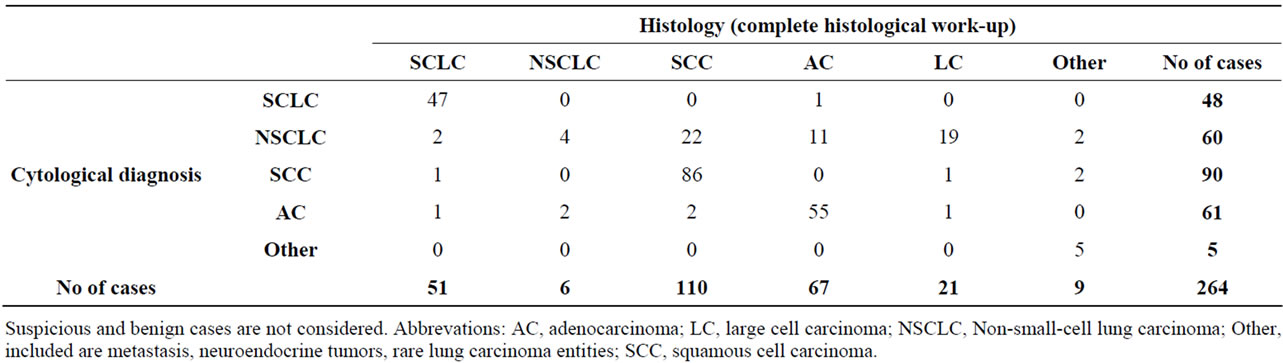
Table 2. Cytological diagnosis in relation to histological diagnosis after complete histological work-up.
not be further subclassified.
Most diagnoses of the 30 LC were established by histology, and in only 5 cases the cytological and histological diagnoses were identical (see Table 2).
4. Discussion
Due to new developments in the field of oncology, the pathological diagnosis of bronchial carcinoma has become more challenging [3,4,16]. According to Travis et al. in daily practice more than 70% of clinically suspected lung cancers are diagnosed by means of small biopsies or cytology [4]. In our department the rate of primary lung cancer diagnosed by lavage specimens in combination with small biopsies, such as FB and CT-guided TT-FNB is even higher (>95%).
BL and bronchial biopsy (FB) are valuable tools in the diagnostic process of lung cancer, but in the literature low sensitivities of washing procedures are reported. With flexible bronchoscopy and BL for central bronchogenic carcinoma the sensitivities range from 31% to 78%, reviewed in [12]. Rennard SI found malignant cells in 69% of bronchoalveolar lavage specimens [17]. Troung et al. reported an overall sensitivity of bronchial washing of 66% [18]. Sensitivity of flexible bronchoscopy combined with BAL in peripheral lesion has been reported to range from 12% to 65%, reviewed in [12]. In our study an overall sensitivity of 83.0% was calculated for both centrally and peripherally located lung tumors and thus rank in the upper range of reported sensitivities. This might probably be due to the specific procedure performed in our departments, since two BL/BAL specimens are obtained and evaluated in association with FB. In contrast to previous studies [14,19-21], the diagnostic yield of BL/BAL before and after FB was statistically significantly higher in the second cytological specimen. The biopsy procedure has probably led to detachment of tumor cells which were then detected in the second BL/BAL. This confirms the importance of frequency of cytologic specimen retrieval and combination of methods [21,22].
Some studies have shown that definitive diagnosis of malignancy was possible by cytology alone. Naryshkin et al., who examined the reliability of bronchoscopic cytology in relation to biopsy, found a rate of 10.7% of nondiagnostic biopsies [23]. Some other studies have demonstrated diagnostic rates of 9.5% and 2.1% for bronchial washing (without histological confirmation), respectively [24,25]. We identified 54/678 (8.0%) cases in which only cytology was diagnostic or generated an abnormal result leading to further investigation. In about 42% of these cases a peripheral located tumor not visible by bronchoscopy and not accessible by FB was present.
As compared with FB, lavage cytology led to a very high rate of 51 false positive diagnoses in our study. Reevaluation showed that in follow-up histological examinations a pulmonary malignancy was proven in 44/51 cases. In 7 remaining cases, inflammatory lesions were found in 3 cases and in 4 cases a diagnostic follow-up was not performed. Taking all of this into consideration, false positivity by cytology occurred in 3/678 (0.4%). In none of these cases unnecessary treatment was administered as the cytology reports were cautiously formulated, and the negative results of the histological examination were adjusted by interdisciplinary review with consideration of additional investigations (e.g. microbiology tests).
Assessment of accuracy of cytological tumor typing was highest in SCLC (97.9%), followed by 95.6% and 90.2% for SCC and AC, respectively. As in the literature, the cytological typing of SCC and SCLC was highly accurate but was less satisfactory for the other types of primary lung carcinomas except AC [18]. Difficulties in cytological tumor typing arose especially in poorly differentiated carcinomas. Other reasons were a low cell number (often seen in BAL/BL samples); bad material preservation and inflammatory background were also problems [26-28]. Another rare problem was the occurrence of small atypical cells presenting difficulties in differentiation of lymphocytes from small cell carcinoma cells or SCLC from small cellular NSCLC, e.g. small cellular SCC. Classification of LC was achieved by histology in the majority of cases. In 5 cases, LC was diagnosed by cytology followed by histology. Most cases of LC had previously been classified as NSCLC by cytology. In fact, it is recommended that LC should not be diagnosed on cytology specimens or small biopsies, in order not to miss an AC for prognostic relevant mutation analysis [29]. Nevertheless, mutation analysis e.g. EGFRgene is also performed on biopsy material of LC diagnosed in our center.
By cytology 60 cases were classified as NSCLC. Respectively, by FB and further histological investigations 14 and 4 NSCLC cases could not be further subclassified. Therefore, our method of lavage cytology before and after FB appears to reduce the diagnostic grey zone of NSCLC significantly to less than 5%. Travis et al. reported 10% - 30% NSCLC, NOS diagnosed by small biopsy and cytology samples [4].
Pulmonary cytopathological methods have excellent sensitivity and specifity in the diagnosis of primary lung carcinomas. Our study shows that the combination of BL/BAL before and after FB can establish the diagnosis of bronchial carcinoma in most cases and allows the subclassification of NSCLC in more than 95% of cases. Additionally, this method is able to establish the diagnosis of pulmonary carcinomas when endoscopic biopsies fail. The performance of double cytological specimen retrival and analysis is particularly helpful in cases in which histopathological diagnosis is hampered by a low tissue yield [18].
5. Acknowledgements
We warmly thank Dr. Ulrike Paulus MD, MRCP, MRCPath for critical reading the manuscript and English revision.
REFERENCES
- M. Parkin, J. E. Tyczynski, P. Boffetta, et al., “Tumours of the Lung,” In: W. D. Travis, E. Brambill, H. K. Muller-Hermelink and C. C. Harris, Eds., World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart, IARC Press, Lyon, 2004, pp. 9-124.
- D. M. Parkin, J. Ferlay, M. P. Curado, F. Bray, B. Edwards, H. R. Shin and D. Forman, “Fifty Years of Cancer Incidence: CI5 I-IX,” International Journal of Cancer, Vol. 127, No. 12, 2010, pp. 2918-2927. doi:10.1002/ijc.25517
- K. M. Kerr, “Pulmonary Adenocarcinomas: Classification and Reporting,” Histopathology, Vol. 54, No. 1, 2009, pp. 12-27. doi:10.1111/j.1365-2559.2008.03176.x
- W. D. Travis, E. Brambilla, M. Noguchi, et al., “International Association for the Study of Lung Cancer/ American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma,” Journal of Thoracic Oncology, Vol. 6, No. 2, 2011, pp. 244-285. doi:10.1097/JTO.0b013e318206a221
- M. Maemondo, A. Inoue, K. Kobayashi, et al., “Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer with Mutated EGFR,” NEJM, Vol. 362, No. 25, 2010, pp. 2380-2388. doi:10.1056/NEJMoa0909530
- T. S. Mok, Y. L. Wu, S. Thongprasert, et al., “Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma,” NEJM, Vol. 361, No. 10, 2009, pp. 947-957. doi:10.1056/NEJMoa0810699
- G. V. Scagliotti, P. Parikh, J. von Pawel, et al., “Phase III Study Comparing Cisplatin plus Gemcitabine with Cisplatin plus Pemetrexed in Chemotherapy-Naive Patients with Advanced-Stage Non-Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 26, No. 21, 2008, pp. 3543-3551. doi:10.1200/JCO.2007.15.0375
- D. H. Johnson, L. Fehrenbacher, W. F. Novotny, et al., “Randomized Phase II Trial Comparing Bevacizumab plus Carboplatin and Paclitaxel with Carboplatin and Paclitaxel alone in Previously Untreated Locally Advanced or Metastatic Non-Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 22, No. 11, 2004, pp. 2184-2191. doi:10.1200/JCO.2004.11.022
- G. Scagliotti, N. Hanna, F. Fossella, et al., “The Differential Efficacy of Pemetrexed According to NSCLC Histology: A Review of Two Phase III Studies,” Oncologist, Vol. 14, No. 3, 2009, pp. 253-263. doi:10.1634/theoncologist.2008-0232
- S. L. Edwards, C. Roberts, M. E. McKean, J. S. Cockburn, R. R. Jeffrey and K. M. Kerr, “Preoperative Histological Classification of Primary Lung Cancer: Accuracy of Diagnosis and Use of the Non-Small Cell Category,” Journal of Clinical Pathology, Vol. 53, No. 7, 2000, pp. 537-540. doi:10.1136/jcp.53.7.537
- J. J. Cataluna, M. Perpina, J. V. Greses, V. Calvo, J. D. Padilla and F. Paris, “Cell Type Accuracy of Bronchial Biopsy Specimens in Primary Lung Cancer,” Chest, Vol. 109, No. 5, 1996, pp. 1199-1203. doi:10.1378/chest.109.5.1199
- G. Schreiber and D. C. McCrory, “Performance Characteristics of Different Modalities for Diagnosis of Suspected Lung Cancer: Summary of Published Evidence,” Chest, Vol. 123, No. 1, 2003, pp. 115-128. doi:10.1378/chest.123.1_suppl.115S
- S. Gasparini, “Bronchoscopic Biopsy Techniques in the Diagnosis and Staging of Lung Cancer,” Monaldi Archives for Chest Disease, Vol. 52, No. 4, 1997, pp. 392- 398.
- M. A. Van der Drift, G. J. van der Wilt, F. B. Thunnissen and J. P. Janssen, “A Prospective Study of the Timing and Cost-Effectiveness of Bronchial Washing during Bronchoscopy for Pulmonary Malignant Tumors,” Chest, Vol. 128, No. 1, 2005, pp. 394-400. doi:10.1378/chest.128.1.394
- H. Motulsky, “Intuitive Biostatistics,” Oxford University Press, Oxford, 1995.
- C. S. Sigel, A. L. Moreira, W. D. Travis, M. F. Zakowski, R. H. Thornton, G. J. Riely and N. Rekhtman, “Subtyping of Non-Small Cell Lung Carcinoma: A Comparison of Small Biopsy and Cytology Specimens,” Journal of Thoracic Oncology, Vol. 6, No. 11, 2011, pp. 1849-1856. doi:10.1097/JTO.0b013e318227142d
- S. I. Rennard, “Bronchoalveolar Lavage in the Diagnosis of Cancer,” Lung, Vol. 168, Suppl. 1, 1990, pp. 1035-1040. doi:10.1007/BF02718241
- L. D. Truong, R. D. Underwood, S. D. Greenberg and J. W. McLarty, “Diagnosis and Typing of Lung Carcinomas by Cytopathologic Methods. A Review of 108 Cases,” Acta Cytologica, Vol. 29, No. 3, 1985, pp. 379-384.
- S. Sompradeekul, U. Chinvetkitvanich, P. Suthinon and S. Wongbunnate, “Difference in the Yields of Bronchial Washing Cytology before and after Forceps Biopsy for Lung Cancer Diagnosis,” Journal of the Medical Association of Thailand, Vol. 89, Suppl. 5, 2006, pp. 37-45.
- H. S. Lee, S. Y. Kwon, D. K. Kim, et al., “Bronchial Washing Yield before and after Forceps Biopsy in Patients with Endoscopically Visible Lung Cancers,” Respirology, Vol. 12, No. 2, 2007, pp. 277-282. doi:10.1111/j.1440-1843.2006.01001.x
- A. Fernández-Villar, A. González, V. Leiro, et al., “Effect of Different Bronchial Washing Sequences on Diagnostic Yield in Endoscopically Visible Lung Cancer,”Archivos de Bronconeumología, Vol. 42, No. 6, 2006, pp. 278-282. doi:10.1016/S1579-2129(06)60143-2
- K. J. Franke, G. Nilius and K. H. Ruhle, “Frequency of Cytological Procedures in Diagnostic Bronchoscopy of Peripheral Pulmonary Modules and Masses,” Pneumologie, Vol. 60, No. 11, 2006, pp. 663-666. doi:10.1055/s-2006-944263
- S. Naryshkin, J. Daniels and N. A. Young, “Diagnostic Correlation of Fiberoptic Bronchoscopic Biopsy and Bronchoscopic Cytology Performed Simultaneously,” Diagnostic Cytopathology, Vol. 8, No. 2, 1992, pp. 119-123. doi:10.1002/dc.2840080206
- V. H. Mak, I. D. Johnston, M. R. Hetzel and C. Grubb, “Value of Washings and Brushings at Fibreoptic Bronchoscopy in the Diagnosis of Lung Cancer,” Thorax, Vol. 45, No. 5, 1990, pp. 373-376. doi:10.1136/thx.45.5.373
- A. M. Jones, I. M. Hanson, G. R. Armstrong and B. R. O’Driscoll, “Value and Accuracy of Cytology in Addition to Histology in the Diagnosis of Lung Cancer at Flexible Bronchoscopy,” Respiratory Medicine, Vol. 95, No. 5, 2001, pp. 374-378. doi:10.1053/rmed.2001.1051
- R. S. Saad and J. F. Silverman, “Respiratory Cytology: Differential Diagnosis and Pitfalls,” Diagnostic Cytopathology, Vol. 38, No. 4, 2010, pp. 297-307.
- M. L. Policarpio-Nicolas and M. R. Wick, “False-Positive Interpretations in Respiratory Cyto-Pathology: Exemplary Cases and Literature Review,” Diagnostic Cytopathology, Vol. 36, No. 1, 2008, pp. 13-19. doi:10.1002/dc.20734
- J. P. Crapanzano and A. Saqi, “Pitfalls in Pulmonary Cytopathology,”Diagnostic Cytopathology, Vol. 39, No. 2, 2011, pp. 144-154. doi:10.1002/dc.21396
- W. D. Travis, “The New IASLC/ATS/ERS International Multidisciplinary Lung Adenocarcinoma Classification. Non-Small Cell Lung Cancer: New Entities and Staging System,” Proceedings of the 8th Atlanta Lung Cancer Symposium, Atlanta, 17th April 2010, pp. 1-60.
Abbreviations
AC: adenocarcinoma;
BAL: bronchial lavage;
BL: bronchial lavage;
CT: computer-tomography;
EGFR: epidermal growth factor receptor;
FB: forceps biopsy;
LC: large cell carcinoma;
NOS: not otherwise specified;
NSCLC: non-small-cell lung carcinoma;
SCC: squamous cell carcinoma;
TT-FNB: trans-thoracic fine-needle biopsy.
NOTES
*Conflict of interest: The authors declare no conflict of interest.
#Corresponding author.

