World Journal of Cardiovascular Diseases
Vol.4 No.9(2014), Article
ID:48740,11
pages
DOI:10.4236/wjcd.2014.49051
A Modified Treatment Failure Classification System with Complete and Exhaustive Accounting of Any Adverse Events Related to the Previous Percutaneous Coronary Intervention
Anders M. Galløe1*, Jørgen L. Jeppesen2, Søren Boesgaard3, John Godtfredsen4, Niels Bligaard5, Jens F. Lassen6, Henning Kelbæk3, Anders Junker7, Jan Ravkilde8, Peter Riis Hansen5, Carsten T. Larsen1, Ghita B. Stephansen3, Ulrik Abildgaard3
1Department of Cardiology, Copenhagen University Hospital, Roskilde, Denmark
2Department of Medicine, Copenhagen University Hospital, Glostrup, Denmark
3Department of Cardiology, Copenhagen University Hospital, Rigshospitalet, Denmark
4Department of Cardiology, Copenhagen University Hospital, Herlev, Denmark
5Department of Cardiology, Copenhagen University Hospital, Gentofte, Denmark
6Department of Cardiology, Aarhus University Hospital, Skejby, Denmark
7Department of Cardiology, Odense University Hospital, Denmark
8Department of Cardiology, Aalborg University Hospital, Denmark
Email: *anders@galloe.dk
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/
![]()
![]()

Received 5 June 2014; revised 7 July 2014; accepted 1 August 2014
ABSTRACT
Objectives: To determine the clinical outcome related to treatment failure of the percutaneous coronary intervention (PCI) itself. Background: When considering the addition of PCI to the medical treatment of angina, it is necessary to know the balance between the benefit and the risk of the PCI itself, but the latter remains unknown. The usual outcome measures are imprecise because they contain events unrelated to the previous PCI and because some events clearly caused by PCI treatment failures are omitted. Methods: In total, 2098 unselected patients were randomized to receive either sirolimus-(n = 1065) or paclitaxel-(n = 1033) eluting coronary stents and followed for five years in the SORT OUT II. Any death, cardiac death, myocardial infarction (MI), stent thrombosis and documented stenosis was classified and combined to a “patient oriented clinical outcome” (POCO), the classical “major adverse cardiac events” (MACE) and the new “PCI-treatment oriented clinical outcome” (TOCO). Results: POCO occurred in 746 patients (35.6%), MACE in 467 patients (22.3%) and TOCO in 293 patients (14.0%), thus TOCO amounted to 39% of the POCO and to 63% of the MACE. Conclusion: By introduction of the present PCI treatment failure classification system, the clinical outcome of PCI-treatment itself may be credulously estimated by the rate of TOCO and eventually PCI is substantially better than what might be perceived from the classically used POCO and MACE rates.
Keywords:PCI, Angina, Clinical Outcome

1. Introduction
When decision has to be made about an eventual addition of a PCI treatment to the medical treatment, knowledge of the balance between benefit and risk of adverse events is necessary to determine if PCI treatment would be advisable. The initial PCI cannot be held responsible for any subsequent adverse event! The clinical outcome related to the PCI-treatment has to concern the actually given lesion or vessel and must not involve other vessels or clinical consequences of disease stemming from untreated vessels.
The Academic Research Consortium (ARC [1] ) has recommended that the clinical outcome after PCI should be estimated by a patient oriented clinical outcome (POCO) measure consisting of adverse events in a combination of all cause death, any MI or any revascularization. The POCO is an unbiased measure with a perspective of what happens to the patient. If the focus is shifted to ask what happens to the PCI treatment itself, then the POCO is a rather imprecise measure. Firstly, because POCO is confounded by irrelevant events like deaths from non-cardiac reasons, cardiac deaths unrelated to the initially treated vessel as well as MI originating from non-treated vessels. Secondly, because some cardiac events are not encountered for in POCO, even though they possess obvious signs of failure of the initial PCI (e.g. in stent re-stenosis if it cannot be treated by revascularization). Consequently, the quintessence of the clinical outcome of the PCI-treatment itself is not reflected by the POCO rate. Attempts to increase the precision by modification of the content of the composite end point have not solved that problem. For instance, the Major Adverse Cardiac Event (MACE) encompassing cardiac death, Myocardial Infarction (MI) and revascularization of the initially treated vessel (Target Vessel Revascularization, TVR) is not merely a measure of treatment failure but is rather reflecting both the treatment failures as well as further progression of atherosclerotic disease in previously untreated lesions as well as MI caused by occlusions of previously untreated vessels.
The target vessel failure rate has been proposed as a measure of efficacy of the PCI treatment itself (ARC [1] ). This composite end point contains target vessel revascularization, death, or myocardial infarction attributed to the target vessel (ARC [1] ), but again this measure is imprecise because death is not necessarily even cardiac and some of the myocardial infarctions with uncertain localization might stem from the treated vessel but such events are not encompassed.
Any outcome measure is imprecise because some events cannot be classified with certainty, but by accounting for each and every possible adverse event, a range between a minimal or definitive and a maximal adverse event rate may be determined.
The purpose of the present article is to present our treatment failure classification system and use it to assess the clinical outcome related to the PCI treatment itself (i.e. the TOCO rate) and report this together with the confidence intervals in conjunction with the commonly known POCO-, MACEand stent thrombosis rates.
2. Methods
The SORT OUT II has previously been thoroughly described [2] [3] . In short, 2098 patients with 2888 lesions were randomized to receive one of the two first commercially available drug eluting stents—the sirolimus-eluting Cypher stent (Cordis/Johnson & Johnson, Miami Lake, Florida) or the paclitaxel-eluting Taxus stent (Boston Scientific Corp, Natick, Massachusetts).
The two groups were well matched at baseline for medication intake, and coronary artery disease [2] .
The main outcome measure in the present context is the rate of TOCO (= definite and probable treatment failure rate (TF-123, Table 1)). The 100% confidence interval spans from definite treatment failure (TF-1) to definite, probable and possible treatment failure (TF-123456).
Secondary outpoint measures were the rates of POCO, MACE and stent thrombosis (= definite and probable stent thrombosis, ST-123, Table 1), the latter with a 100% confidence interval ranging from definite stent thrombosis (ST-1) to definite, probable and possible stent thrombosis (ST-12345) which includes, as a new item: MI with uncertain localization.
2.1. The Treatment Failure Classification System
The events not previously accounted for in the traditional clinical outcome measures are 1) angiographically proven re-stenosis in the initially treated lesion if it is left untreated by revascularization (for whatever reason); 2) proven stenosis in the initially treated vessel but outside the initially treated lesion, again if it is left un-treated by revascularization (such a stenosis may have been caused by injury from gear used during the initial PCI and may therefore be classified as a possible treatment failure) and 3) MI with uncertain localization (it may still potentially be an in stent thrombosis, but as it is impossible to determine the localization of the MI in relation to the initially treated vessel it is not encompassed in the stent thrombosis classification system from ARC [1] .
The events often included, even though they contain elements unrelated to the initial PCI, are 1) all cause death which contains non-cardiac death; 2) MI which contains MI in areas irrigated by other coronary vessels than the initially treated vessel; 3) cardiac death which contains some cardiac deaths with a cause being unrelated to the initial PCI (e.g. death due to a new MI in a previously untreated vessel).
The treatment failure classification system was constructed to consist of one single mutual outcome measure that combined POCO, stent thromboses and angiographically proven re-stenoses in the treated lesions as well as stenoses in the treated vessels. It is an exhaustive classification system because it is making account of any death, any cardiac event and any sign of treatment failure that cannot be rejected as being related to the initial PCI.
At the same time it is rejecting events caused by death of non-cardiac reasons as well as cardiac events unrelated to the initial treated vessel. Consequently it is a more precise estimation of treatment failure of the initial PCI and thereby a measure of the clinical outcome of the PCI treatment itself.
The complete treatment failure classification system have several different sub-classes and is closely related to the classification of definite, probable or possible stent thrombosis known from ARC [1] . Likewise, we defined end points with varying degree of certainty of a relation to the initially treated vessel (including the initially treated lesion). It is ranging from a definitely proven relation to a definitely rejected relation (Table 1). Several events cannot be exactly determined to be connected to the initially treated vessel and consequently the genuine treatment failure rate remains unknown. A minimal and a maximal rate of treatment failures are easily obtainable and they will define the boundaries for a complete (or 100%) confidence interval for the presumed genuine treatment failure rate. This rate must be at least the rate of definitely proven treatment failure (TF-1, Table 1). With increasing treatment failure numbering (TF-2, TF-3, TF-4, TF-5, TF-6, Table 1) there is a decreasing likelihood of a connection between the initially treated vessel and a subsequent cardiac event. The theoretical maximal treatment failure rate is estimated by counting any cardiac event or subsequent stenosis that could not with certainty be rejected as related to the initially treated vessel. Consequently, the rate of presumed genuine treatment failure is beyond the minimal treatment failure rate (TF-1, Table 1) but below the theoretically maximal treatment failure rate (TF-123456, Table 1). Rejected or definitely no treatment failure is classified as TF-7 in order to enable complete accounting of all detected adverse events: any death, any MI, any stent thrombosis, any revascularization and any coronary angiography (Table 2).
2.2. Statistical Analyses
All analyses were performed according to the intention-to-treat principle. All p-values are two sided with a significance level of 0.05. The cumulative proportion of patients experiencing an event was analyzed according to the date of the first event for each patient and depicted in Kaplan-Meier plot. We performed log-rank test to assess the p-value. If patients emigrated, the date of emigration was used as censoring date. Death for any reason and POCO was censored for emigration; cardiac death and MACE was censored for emigration and non-cardiac death. Other end points were censored for death and emigration.
Table 1. The PCI treatment failure classification system.
The treatment failure (TF) classification and the corresponding stent thrombosis classification abbreviated from Table 7 from the Academic Research Consortium (ARC). The events are related to the initially treated lesion or vessel and classified according to the TF-numbering. With increasing TF-number, the likelihood of a relation to the initial PCI is decreasing. The TF-classification is combining and classifying each and every death, cardiac death, myocardial infarction (MI), stent thrombosis (ST) and stenosis in initially treated lesion or in initially treated vessel whether or not it is treated by revascularization. The seven classes enable full accounting of all adverse events into one single mutual and exhaustive classification system. Re-stenosis in the initially treated lesion is obviously a treatment failure and is included in TF-1 even if it is not treated by revascularization. This is opposed to previously known clinical outcome measures. Likewise, a de novo stenosis outside the initially treated lesion but inside the initially treated vessel is included in TF-6. The stent thrombosis classification proposed by ARC has been expanded by a new class (ST-5): a MI with uncertain localization, because this might still be an in stent thrombosis. TF-7 contains events where the connection to the initial PCI is rejected. Abbreviations: TLR (target lesion revascularization), PCI (percutaneous coronary intervention); CABG (coronary artery bypass grafting), TVR (target vessel revascularization), MACE (major adverse cardiac event).
Table 2. The number (and percentages) of patients experiencing an event. The percentages are the arithmetical ones (the actual number of events divided by the total number in the head row); in brackets [the censored percentages]. The p values are calculated from Log-rank test of cumulated event rates and are based on censored observations.
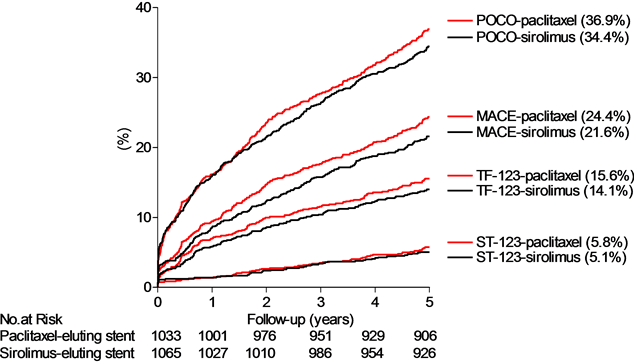
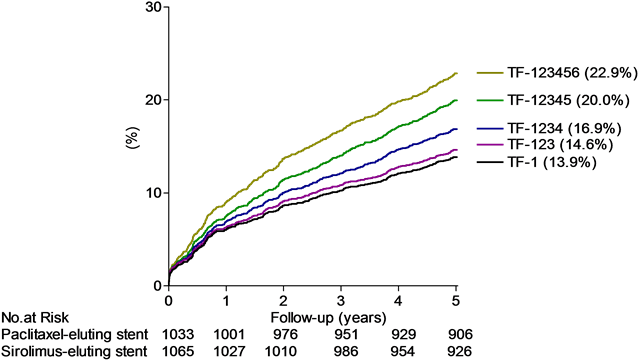
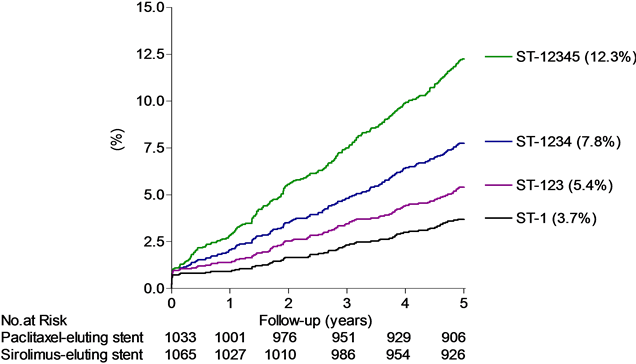
Figure 1. Top panel: The cumulative proportions of patients (%) experiencing an event detailed for POCO, MACE, TOCO (the definite and probable treatment failure (TF-123) equal to the presumed genuine treatment failure) and the definite and probable stent thrombosis (equal to the presumed genuine stent thrombosis (ST-123)) by time (years), with sirolimus-(red lines) or paclitaxel-(black lines) eluting stent groups. Middle panel: The cumulative proportions of patients (%) experiencing the different treatment failure classes by time (years). Bottom panel: The cumulative proportions of patients (%) experiencing the different stent thrombosis classes by time (years), detailed for definite stent thrombosis (ST-1), definite and probable stent thrombosis (presumed genuine stent thrombosis rate (ST-123)), definite, probable and possible stent thrombosis (ST-1234, ARC definitions) and the maximal stent thrombosis rate (ST-12345, our own definition (Table 1)). Below abscissa is shown the number at risk (no. at risk).
3. Results
The number of patients with an event is listed in Table 2 and the event rates for the principal outcome measures are depicted in Figure 1, top panel. There were no statistical significant differences in any of the combined or individual event rates between sirolimusand paclitaxel-eluting stent groups (Table 2). The rates of POCO, MACE, TOCO (=definite and probable treatment failure, TF-123) and stent thrombosis (=definite and probable stent thrombosis, ST-123) within 5 years were 35.6%, 22.3%, 14.0% and 5.1% respectively.
The TOCO (TF-123) was detected in 293 (14.0%) of the patients with a complete (100%) confidence interval ranging from at least 13.2% (TF-1) to at most 22.0% (TF-123456, Table 2, Figure 1 middle panel). The rate of TOCO (=TF-123) amounted to 39% of the POCO rate and to 63% of the MACE rate.
The presumed genuine stent thrombosis is reflected in the definite and probable stent thrombosis rate (ST-123, Table 1) and was detected in 107 (5.1%) of the patients with a complete (100%) confidence interval ranging from at least 73 (3.5%) (ST-1) to at most 246 (11.7%) (ST-12345, Table 1, Figure 1 bottom panel). The rate of definite and probable stent thrombosis (ST-123) amounted to 14% of the POCO rate, 23% of the MACE rate and to 36% of the TOCO rate (TF-123).
Additional Analysis
We also thought it important to report the total number of revascularization as well as revascularizations caused by treatment failure. Any subsequent revascularization with either PCI or coronary artery bypass grafting (CABG) occurred in 487 patients (23.2%). Revascularization of the initially treated lesion (target lesion revascularization, TLR) occurred in 224 patients (10.7%). Revascularization caused by stenosis outside the initial treated lesion but still inside the same vessel occurred in 60 patients, and consequently TVR occurred in 284 patients (13.5%). Thus TLR amounted to 46.0% and TVR (including TLR) to 58.3% of all subsequent revascularizations (Figure 2).
Among the 224 patients with subsequent TLR, 192 patients experienced one revascularization, 26 had two, 5 had three, and 1 had four revascularizations.
Among the 284 patients with subsequent TLR or TVR, 244 patient experienced only one revascularization, 32 had two, 7 had three, 0 had four and 1 had five revascularizations. Thus 40 patients of the entire study population of 2098 patients (1.9%) required at least two subsequent TLR or TVR.
4. Discussion
The principal finding was that the definite and probable treatment failure or TOCO rate after five years was 14.6% even after inclusions of adverse events traditionally left out. Initially we selected the rate of definite and probable treatment failure (TF-123, Table 1) to represent a guess of the presumed genuine treatment failure rate. This
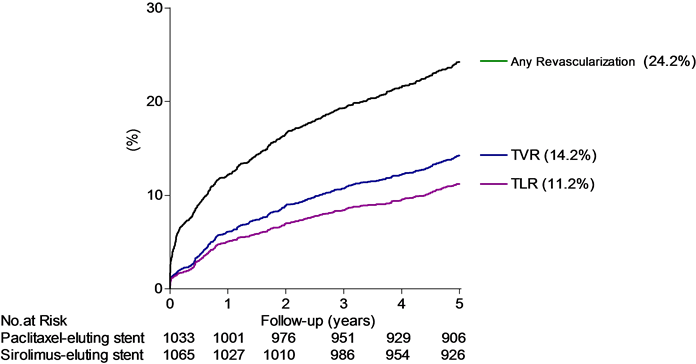
Figure 2. Cumulated proportion of patients (%) experiencing a subsequent revascularization with either CABG or PCI by time (years). The TLR rate amounted to 46.3% and TVR (including TLR) to 58.7% of all revascularizations. Below abscissa is shown the number at risk (no. at risk).
was chosen because it corresponded to the presumed genuine stent thrombosis which is identical to the definite and probable stent thrombosis according to the ARC definitions (identical to ST-123, Table 1). Any choice will not be exactly correct because it will contain events that are not treatment failures and it may lack events that would in fact have been classified as treatment failures if better elucidation were possible. The TOCO rate (TF-123) only represents one guess on the true and genuine treatment failure of the initial PCI. The quality of this guess may be estimated by two other results. First the fact that 58.3% of any subsequent revascularizations were TLR or TVR. Second, the result from the PROSPECT study where “only” approximately half of all subsequent adverse events were related to the initial PCI [4] . Parallel to these figures we found a TOCO rate of 63% of the MACE rate. It shall again be emphasized, that our measure includes events that are traditionally left out. These two results support that the TOCO rate (TF-123) may serve as a measure that is obviously also reflecting the real rate of genuine treatment failure. The TOCO rate (TF-123) is still only a guess and our choice must therefore be accompanied by a (100%) confidence interval, which is enabled by the way the treatment failure classification is constructed.
Regularly revisions occur in the consensus on how to evaluate the efficacy and safety of PCI. This is reflected in the frequent changes of the endpoint definitions and the discussions of the proper way to report them [1] [5] [6] . In line with the suggestions from ARC on how to report outcome, we explicitly report all treatment failure classes. The corresponding depiction of all treatment failure curves enables the reader to choose an alternative and personalized selection of a curve thought to be better reflecting the presumed genuine treatment failure rate (Figure 1, middle panel). The estimation of a presumed genuine stent thrombosis rate likewise needs a confidence interval, and by the new inclusion of MI with uncertain localization (=ST-5) we have enabled determination of a 100% confidence interval for stent thrombosis (Figure 1, bottom panel).
With the use of our exhaustive treatment failure classification system in future PCI studies it will be possible for others to repeat our analyses of treatment failure rates and of the fraction of any revascularizations that are TLR or TVR and thereby help qualifying whether the TOCO rate (TF-123) is the best reflection of the real—and not only the presumed—genuine treatment failure rate. Until then, we found the rate of TOCO to be in the vicinity of 40% of the unbiased POCO rate and 60% of the MACE rate, and we believe it is important to remember these approximate fractions when evaluating the genuine clinical effect of the PCI itself from POCO or MACE reported in other studies.
4.1. Clinical Consequences of the Treatment Failure Classification
The treatment of coronary artery stenoses with PCI reached new effectiveness with the introduction of the drug-eluting stents [7] . Five years after their introduction into routine clinical practice, the COURAGE study showed that the addition of PCI to optimal medical therapy did reduce the number of angina attacks by 40% [7] , but in fact did not change mortality or acute MI rates [8] . Despite various interpretations of the COURAGE results, including that the study predominantly was a bare metal stent study, the use of PCI in the United States promptly fell by 26% in patients with stable angina [9] . Four years later the PROSPECT study showed that only around half of the MACE could be ascribed to the initially treated vessel while the other half of the MACE rate stemmed from progression of disease or new lesions in other coronary vessels [4] —essentially adverse events that the initial PCI cannot be held responsible for.
In a recently published meta-analysis of 12 randomized studies comparing optimal medical therapy with or without additional PCI (n = 7182) it was reported, that the HR for mortality in the PCI group was 0.85 when compared to the group receiving optimal medical therapy [10] . The confidence interval was 0.71 - 1.01 and thus obviously statistically non-significant [11] . These figures were reported as intention to treat results and as some patients crossed over to PCI after randomization, the real beneficial effect of PCI may be underestimated! However, the null hypothesis was not rejected and as a consequence it was concluded that there was no difference in mortality. There may tough be other alternative hypotheses than exactly the null hypothesis itself and—at least—it seems fair to state, that PCI is not likely to increase mortality. As PCI treatment was associated with a non significant but lower MI rate (HR = 0.93) and concomitantly had a significantly increased rate of total freedom from angina (HR = 1.20) (not only relieved symptoms) we believe it is fair to conclude, that PCI is a good and safe therapy [10] . Judged from the TOCO rate PCI is a treatment better than what may be perceived from the MACE or POCO rate. Our data showed that the TOCO rate amounted to 2.5% during the initial PCI hospitalization and 2.5% each year thereafter, with only a few of the initially treated patients needing at least two subsequent revascularization (n = 40 or 1.9%) in the initially treated vessel. This also supports the notion that PCI is safe and effective and may be offered to the vast majority of patients experiencing symptoms of angina and not only be reserved to the patients with “unacceptable angina” as otherwise recommended in the former and the latest ACCF/AHA/SCAI Guidelines [12] [13] .
4.2. The Durability of PCI with Drug Eluting Stents
The definite treatment failure class (TF-1) encompasses not only TLR and definite stent thrombosis but also proven re-stenosis left untreated. Freedom from definite treatment failure (TF-1) is therefore a PCI analogue to the surgically known graft patency after CABG and it may therefore be used to estimate the durability of the PCI treatment. In our unselected patients, the TF-1 rate was 13.2% and freedom from TF-1 is correspondingly 86.8%. Interestingly, the five years patency rate have been reported to be 72% of Saphenous vein grafts and 88% - 92% for internal mammary artery grafts [14] [15] . In plain words, PCI with a drug eluting stent appears to have almost the same durability as mammary grafts after five years observation.
4.3. Limitations
It is a major limitation, that there is no way to find the real treatment failure rate. We have only indirect estimations. We find the POCO rate to be very useful to estimate what the patient may face in unbiased total health risk for the future but regrettably this combined end point is seldom reported, even in publications referring to ARC. The coupling of the rate of TOCO to be a fixed percentage of the POCO or the MACE rate is rather imprecise, but it may be a solution to estimate the rate of TOCO conditioned that our present findings are reproduced in other patient populations.
5. Conclusions
The treatment failure classification contains an exhaustive accounting of deaths, MI and revascularizations together with stent thromboses, re-stenoses and de-novo stenoses. These adverse events are combined into one single mutual classification system. From this, it is possible to extract unwanted events seen from the patient perspective (POCO), seen from a cardiac perspective (MACE) or seen from a perspective of the PCI itself (TOCO). The latter is not neglecting any treatment failures but rejects cardiac events definitely unrelated to the initially treated vessel and it is consequently producing a more complete and exhaustive accounting of the clinical outcome of the PCI itself. The rates of POCO, MACE, TOCO (TF-123) and stent thrombosis (ST-123) within 5 years were 35.6%, 22.3%, 14.0% and 5.1% respectively. The clinical consequence is that the clinical effect of PCI itself is better than what is reflected in the total POCO or MACE rate.
In clinical situations where CABG is not mandatory, PCI does certainly not increase mortality based on the available medical literature and may thus be used in a less reluctant way and not only be reserved to clinical situations where symptoms are “unacceptable” as otherwise recommended.
The present extraction of TOCO rate give a more complete estimate of the effect of the PCI itself and may be utilized in the next revision of ACCF/AHA/SCAI guideline—hopefully to the benefit of the many patients who are symptomatic with “acceptable angina” but would potentially be relieved from any cardiac symptom by a successful PCI treatment.
Acknowledgements
The Danish Heart Registry (DHR) has contributed with essential detection of invasive cardiac procedures.
Funding/Support
Boston Scientific and Cordis, a Johnson & Johnson company donated unrestricted research grants to support completion of the study, event detection, and classification for a total follow-up period of five years.
Role of the Sponsors
Boston Scientific and Cordis had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
References
- Cutlip, D.E., Windecker, S., Mehran, R., et al. (2007) Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions. Circulation, 115, 2344-2351.
- Galloe, A.M., Thuesen, L., Kelbaek, H., et al. (2008) Comparison of Paclitaxeland Sirolimus-Eluting Stents in Everyday Clinical Practice: The SORT OUT II Randomized Trial. JAMA, 299, 409-416.
- Bliggard, N., Thuesen, L., Saunamaki, K., et al. (2014) Similar Five-Year Outcome with Paclitaxeland SirolimusEluting Coronary Stents. Scandinavian Cardiovascular Journal, 48, 148-155.
- Stone, G.W., Maehara, A., Lansky, A.J., et al. (2011) A Prospective Natural-History Study of Coronary Atherosclerosis. The New England Journal of Medicine, 364, 226-235.
- Mauri, L., Hsieh, W.H., Massaro, J.M., Ho, K.K., D’Agostino, R. and Cutlip, D.E. (2007) Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents. The New England Journal of Medicine, 356, 1020-1029.
- Kip, K.E., Hollabaugh, K., Marroquin, O.C. and Williams, D.O. (2008) The Problem with Composite End Points in Cardiovascular Studies: The Story of Major Adverse Cardiac Events and Percutaneous Coronary Intervention. Journal of the American College of Cardiology, 51, 701-707.
- De, L.G., Dirksen, M.T., Spaulding, C., et al. (2012) Drug-Eluting vs Bare-Metal Stents in Primary Angioplasty: A Pooled Patient-Level Meta-Analysis of Randomized Trials. Archives of Internal Medicine, 172, 611-621.
- Boden, W.E., O’Rourke, R.A., Teo, K.K., et al. (2007) Optimal Medical Therapy with or without PCI for Stable Coronary Disease. The New England Journal of Medicine, 356, 1503-1516.
- Ahmed, B., Dauerman, H.L., Piper, W.D., et al. (2011) Recent Changes in Practice of Elective Percutaneous Coronary Intervention for Stable Angina. Circulation: Cardiovascular Quality and Outcomes, 4, 300-305.
- Pursnani, S., Korley, F., Gopaul, R., et al. (2012) Percutaneous Coronary Intervention versus Optimal Medical Therapy in Stable Coronary Artery Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Circulation: Cardiovascular Interventions, 5, 476-490.
- Gliner, J.G.J., Leech, N.L.N. and Morgan, G.M.G. (2002) Problems with Null Hypothesis Significance Testing (NHST): What Do the Textbooks Say? Journal of Experimental Education, 71, 83-92.
- Levine, G.N., Bates, E.R., Blankenship, J.C., et al. (2011) ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Journal of the American College of Cardiology, 58, e44-e122.
- Fihn, S.D., Gardin, J.M., Abrams, J., et al. (2012) ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology, 60, e44-e164.
- Goldman, S., Zadina, K., Moritz, T., et al. (2004) Long-Term Patency of Saphenous Vein and Left Internal Mammary Artery Grafts after Coronary Artery Bypass Surgery: Results from a Department of Veterans Affairs Cooperative Study. Journal of the American College of Cardiology, 44, 2149-2156.
- Shah, P.J., Durairaj, M., Gordon, I., et al. (2004) Factors Affecting Patency of Internal Thoracic Artery Graft: Clinical and Angiographic Study in 1434 Symptomatic Patients Operated between 1982 and 2002. European Journal CardioThoracic Surgery, 26, 118-124.
Abbreviations
ARC—Academic Research Consortium POCO—Patient Oriented Clinical Outcome PCI—Percutaneous Coronary Intervention MACE—Major Adverse Cardiac Events MI—Myocardial Infarction TVR—Target Vessel Revascularization TOCO—PCI-Treatment Oriented Clinical Outcome TF—Treatment Failure
NOTES
![]()
*Corresponding author.


