Open Journal of Gastroenterology
Vol.09 No.01(2019), Article ID:89908,6 pages
10.4236/ojgas.2019.91001
Efficacy and Safety of Glecaprevir/Pibrentasvir in Combination Therapy in Chronic Hemodialysis Patients with Genotype 2 Hepatitis C Virus Infection
Department of Internal Medicine, Division of Hepatology and Gastroenterology, Masuko Memorial Hospital, Aichi, Japan
Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 24, 2018; Accepted: January 12, 2019; Published: January 15, 2019
ABSTRACT
Background: Glecaprevir (nonstructural protein 3/4A protease inhibitor) and Pibrentasvir (nonstructural protein 5A inhibitor) (G/P), a coformulated once-daily, all oral, ribavirin (RBV)-free, direct-antiviral regimen, was evaluated for safety and efficacy in chronic hemodialysis patients with genotype 2 hepatitis C virus infection. Methods: In this prospective, observational, single-center study at Masuko Memorial Hospital, between November 2017 and December 2018, a total of 8 HD patients with an HCV infection genotype 2 received G/P combination therapy. Age was an average of 67.1 (61 - 75) years and there were four men and two women. It was FIB4 INDX an average of 2.67 (1.5 - 3.34) before the start of therapy. It was quantity of HCV RNA an average of 4.43 (2.1 - 6.5). HCV RNA levels were measured by real-time RCR-based method (COBAS AmpiPrep/COBAS TaqMan HCV Test. 4 cases 12 weeks were 2 cases eight weeks for dosing period. Patients were excluded if they had evidence of hepatocellular carcinoma. This study was approved by the ethics committee of our hospital, while we obtained written consent from the participants after providing a thorough explanation of the contents and methods of this study. Results: 6 patients were available for total dose internal use. As for the HCV RNA of the fourth week, (100%) HCV RNA became negative after administration start of therapy. Rapid virologic response (RVR) achieved all cases. 5 patients achieved 12-week sustained virologic response (SVR12) and were following up the 1 patient. The itching appeared in two cases (33%), but there was symptom improvement in nalfurafine hydrochloride use treatment, and treatment continuation was possible. Conclusion: It is thought that G/P can be given to the HD patients’ safety, but we will accumulate a case in future, and it is thought to be necessary to examine utility and safety.
Keywords:
HCV, RNA, Hepatitis C, Virus Infection, Chronic Hemodialysis, Patients

1. Introduction
Hepatitis C virus (HCV) infects more than 170 million people worldwide and causes liver cirrhosis and hepatocellular carcinoma. Thus, HCV infection is an important health concern [1] [2] . Recent studies have clearly revealed that the prognosis of hemodialysis (HD) patients with an HCV infection is significantly worse compared with dialysis patients not infected with HCV [3] . Death form hepatocellular carcinoma (HCC) and cirrhosis or liver failure is markedly higher in hemodialysis patients with HCV infection than non-infected patients with end-stage renal disease (ESRD) [4] . Patients with chronic kidney disease (CKD) on renal replacement therapy especially HD continue to have a higher prevalence of hepatitis C virus infection than the general population. The prevalence of anti-HCV seropositivity in patients undergoing regular dialysis in developed countries ranges between 7% and 40% [5] [6] [7] .
Recently, the Kidney disease Improvement Global Outcome (KIDIGO) and Japanese Society for Dialysis Therapy (JSDT) established the Guideline of Treatment of Hepatitis C Virus infection in dialysis patients [8] [9] . Glecaprevir (nonstructural protein 3/4A protease inhibitor) and Pibrentasvir (nonstructural protein 5A inhibitor)(G/P), a coformulated once-daily, all oral, ribavirin (RBV)-free, direct-antiviral regimen, was evaluated for safety and efficacy in chronic hemodialysis patients with genotype 2 hepatitis C virus infection.
2. Methods
Single-center study at Masuko Memorial Hospital, between November 2017 and December 2018, a total of 8 HD patients showed in Table 1 with an HCV infection genotype 2 received G/P combination therapy. Age was an average of 67.1 (61 - 75) years and was four men, women two. It was FIB4 INDX an average of 2.67 (1.5 - 3.34) before the start of therapy. It was quantity of HCV RNA an average of 4.43 (2.1 - 6.5). 4 cases 12 weeks were 2 cases eight weeks for dosing period. Patients were excluded if they had evidence of hepatocellular carcinoma. This study was approved by the ethics committee of our hospital, while we obtained written consent from the participants after providing a thorough explanation of the contents and methods of this study.
3. Results
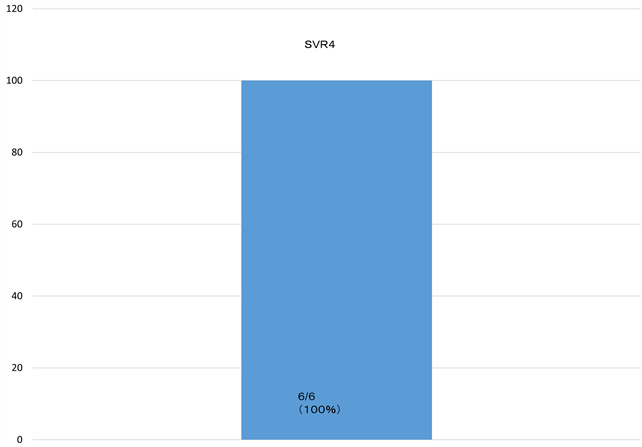
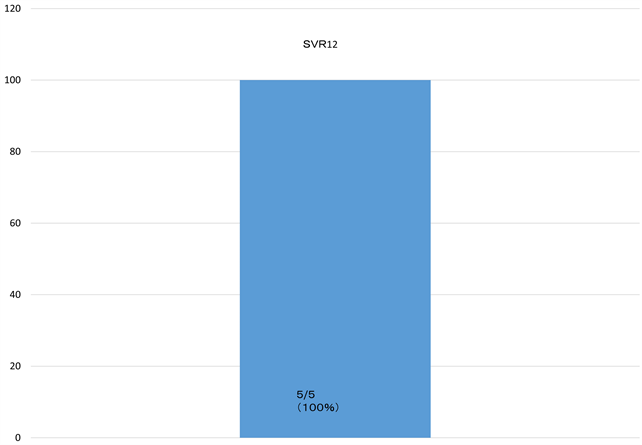
6 patients were available for total dose internal use. 6 patients were available for total dose internal use. As for the HCV RNA of the fourth week, (100%) HCV RNA became negative after administration start of therapy. Rapid virological response (RVR) achieved all cases (Figure 1). 5 patients achieve SVR12 and are following up the 1 patient (Figure 2).
The itching appeared in two cases (33%), but there was symptom improvement
Figure 1. As for the HCV RNA of the fourth week, (100%) HCV RNA became negative after administration start of therapy. Rapid virological response (RVR) achieved all cases.
Figure 2. 5 patients achieve rates of sustained virologic response 12 weeks after treatment (SVR12) and are following up the 1 patient.
Table 1. Patient demographic data.
in nalfurafine hydrochloride use treatment, and treatment continuation was possible.
4. Discussion
In this study, to our knowledge, we are the first to report the effectiveness and safety of G/P combination therapy in chronic hemodialysis patients with genotype 2 hepatitis C virus infection. The genotype 1 report appears, but the report in genotype 2 patients on dialysis is the first time.
Kikuchi et al. report the interferon therapy of the patient on dialysis, but an SVR rate is 39% (22 of 56) in a report of REACH Study by peg INFα2a, [10] that the subject limited to the case that there were few young people and complications without being able to say the enough SVR rate. In addition, the SVR rate of Peg-interferon monotherapy is not high and significant rate of adverse events have been observed. Several oral direct-antiviral drugs (DAAs) for HCV have recently been developed.
An oral agent of the interferon-free (daclatasvir (DCV) and asunaprevir (ASV)) becomes the initiation for the next 2106 years. Indeed, a previous study reported no significant in pharmacokinetics of two drugs between patients with ESRD receiving hemodialysis and those without renal dysfunction. Therefore, theses DAAs may be used in patients who are undergoing hemodialysis, with high anti-HCV efficacy expected. Toyoda et al. also reported the efficacy and safety of daclatasvir/asunaprevir combination therapy for HCV genotype 1b-infeted dialysis patients (overall SVR 12 rate 100%: 28/28), and treatment-related adverse events were similar to those noted among patients with normal renal function [11] ; Suda et al. [12] . Over all, total of 95.5% (20/21) SVR12 of the patients achieved SVR12. However, DAAs did not have the adaptation to the genotype 2 patients.
Atsukawa et al. retrospectively analyzed the safety and efficacy of paritaprevir/ritonavir and ombitasvir in 31 hemodialysis patients with genotype1b infrction. The overall SVR12 rate was 96.8% (30/31). Eleven patients (35.5%) experienced adverse events (AEs). One patient discontinued this combination therapy due to AEs and experienced virological relapse [13] . Also, ribavirin (RBV) can be used with dosage modifications in patients with impaired renal function, the majority of HCV infected patients with ESRD have gone untreated.
Paul et al. 20 reported that patients completed 12 weeks of treatment eighteen of the 20 patients achieved SVR12 [14] . Currently, no HCV treatment for genotype 2 is approved in Japan for duration of less than 12 weeks or without RBV. The elimination of RBV from DAA regimens can improve tolerability and reduce rates of treatment discontinuation due to AEs as RBV is associated with decreases in hemoglobin and elevations of indirect bilirubin.
G/P a coformulated once-daily, all oral, ribavirin (RBV)-free, direct-antiviral regimen, was evaluated for safety and efficacy in chronic hemodialysis patients with genotype 2 hepatitis C virus infection [15] [16] .
Oral medicine was able to be given in 6 cases all cases in the examination in this hospital during an adaptation period, respectively. When we used interferon, we might stop treatment for anemia update and leukopenia, but we took G/P without it looking like it and were able to continue. Also, two patients had itching without showing the side effects such as anorexia or the general malaise. Internal use continuation was enabled in acknowledgment of the relief of symptom by using nalfurafine hydrochloride as correspondence to these cases. There is little number of cases, but we will accumulate a case in future and want to examine safety and efficacy now.
5. Conclusion
It is thought that G/P can be given to the HD patients safely, but we will accumulate a case in future, and it is thought to be necessary to examine utility and safety.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Hotta, N. (2019) Efficacy and Safety of Glecaprevir/Pibrentasvir in Combination Therapy in Chronic Hemodialysis Patients with Genotype 2 Hepatitis C Virus Infection. Open Journal of Gastroenterology, 9, 1-6. https://doi.org/10.4236/ojgas.2019.91001
References
- 1. Mohd Hanafiah, K., Groeger, J., Flaxman, A.D., et al. (2013) Global Epidemiology of Hepatitis C Virus Infection: New Estimates of Age-Specific Antibody to HCV Seroprevalence. Hepatology, 57, 1333-1342. https://doi.org/10.1002/hep.26141
- 2. Seff, L.B. (2002) Natural History of Chronic Hepatitis C. Hepatology, 36, S35-S46.
- 3. Fabrizi, F., Takkouche, B., Lunghi, G., et al. (2007) The Impact of Hepatitis C Virus Infection on Survival in Dialysis Patients: Meta-Analysis of Observational Studies. Journal of Viral Hepatitis, 14, 697-703.
- 4. Nakayama, E., Akita, T., Marumo, F., et al. (2000) Prognosis of Anti-Hepatitis C Virus Antibody-Positive Patients on Regular Hemodialysis Therapy. Journal of the American Society of Nephrology, 11, 1896-902.
- 5. Finelli, L., Miller, J.T., Tokars, J.I., Alter, M.J. and Arduino, M.J. (2005) National Surveillance of Dialysis-Associated Diseases in the United States, 2002. Seminars in Dialysis, 18, 52-61.
- 6. Fissell, R.B., Bragg, G., Woods, J.D., et al. (2004) Patterns of Hepatitis C Prevalence and Seroconversion in Hemodialysis Units from Three Continents: The DOOPS. Kidney International, 65, 2335-2342. https://doi.org/10.1111/j.1523-1755.2004.00649.x
- 7. Jadoul, M., Poignet, J.-L., Geddes, C., et al. (2004) The Changing Epidemiology of Hepatitis C Virus (HCV) Infection in Haemodialysis: European Multicentre Study. Nephrology Dialysis Transplantation, 19, 904-909. https://doi.org/10.1093/ndt/gfh012
- 8. Akiba, T., Hora, K., Imawari, M., et al. (2011) 2011 Japanese Society for Dialysis Therapy Guidelines for the Treatment of Hepatitis C Virus Infection in Dialysis Patient. Journal of the Japanese Society for Dialysis Therapy, 44, 481-531.
- 9. Diseas, K. (2008) Improving Global KDIGO Clinical Practice Guidelines for the Prevention, Diagnosis Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney International Supplements, 73, S1-S99.
- 10. Kikuchi, K., Akiba, T., Nitta, K., et al. (2014) Multicenter Study of Pegylated Interferon α-2a Monotherapy for Hepatitis C Virus-Infected Patients on Hemodialysis: REACH Study. Therapeutic Apheresis and Dialysis, 18, 603-611. https://doi.org/10.1111/1744-9987.12189
- 11. Toyoda, H., Kumada, T., Tada, T., et al. (2016) Safety and Efficacy of Dual Direct-Acting Antiviral Therapy (Daclatasvir and Asunaprevir) for Chronic Hepatitis C Virus Genotype 1 Infection in Patients on Hemodialysis. Journal of Gastroenterology, 51, 741-747. https://doi.org/10.1007/s00535-016-1174-4
- 12. Suda, G., Kudo, M., Nagasaka, A., et al. (2016) Efficacy and Safety of Daclatasvir and Asunaprevir Combination Therapy in Chronic Hemodialysis Patients with Chronic Hepatitis C. Journal of Gastroenterology, 51, 733-740. https://doi.org/10.1007/s00535-016-1162-8
- 13. Arai, T., Atsukawa, M., Tsubota, A., et al. (2018) Efficacy and Safety of Ombitasvir/Paritaprevir/Ritonavir Combination Therapy for Genotype 1b Chronic Hepatitis C Patients Complicated with Choric Kidney Disease. Hepatology Research, 48, 549-555. https://doi.org/10.1111/hepr.13058
- 14. Paul, P., Rajender, R., Parvez, S., et al. (2016) Efficacy of Direct-Acting Antiviral Combination for patients With Hepatic C Virus Genotype 1 Infection and Severe Renal Impairment of End-Stage Renal Disease. Gastroenterology, 150, 1590-1598.
- 15. Edward, G., Eric, L., David, P., et al. (2017) Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. The New England Journal of Medicine, 12, 1448-1455.
- 16. Toyoda, H., Chyayama, K., Suzuki, F., et al. (2017) Efficacy and Safety of Glecaprevir/Pibrentasvir in Japanese Patients With chronic Genotype 2 Hepatitis C Virus. Infection Hepatology, E-Pub Ahead Print.