Open Journal of Organic Polymer Materials
Vol.3 No.1(2013), Article ID:27039,5 pages DOI:10.4236/ojopm.2013.31001
Inorganic Molecularly Imprinted Polymer by Sol-Gel Process for Recognition of Caffeine
1Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
2Macromolecular Science and Engineering, University of Michigan, Ann Arbor, USA
3Department of Chemistry, Chungbuk National University, Cheongju, Korea
Email: *jsshin@chungbuk.ac.kr
Received September 15, 2012; revised October 20, 2012; accepted November 5, 2012
Keywords: Molecularly Imprinted Polymer; Sol-Gel Process; Caffeine; Theophylline
ABSTRACT
A molecularly imprinted polymer (MIP) was formed using an inorganic polymer by a sol-gel process. The monomers which were used to synthesize the inorganic polymer were tetraethoxysilane (TEOS), triethoxymethylsilane (MTES), and triethoxyphenylsilane (PTES). Caffeine was chosen as a template for the molecular imprinting, and theophylline was chosen as the analogous counterpart compound. The discriminating ability of the synthesized MIP to these twocompounds was estimated in this study. The MIP showed the highest discriminating ability when the ratio of TEOS:MTES:PTES in the synthesis of the inorganic polymer was 1:1:3, the reaction temperature was 50˚C, and the pH of the reaction system was ~6.5.
1. Introduction
Recently, significant attention has been paid to the development of a molecular imprinting technique that enables the production of polymers which mimic biological receptors. This technique is a very useful approach for the fabrication of a matrix with molecular recognition sites that are formed by the addition of template molecules during the matrix formation process, and for the removal of the template molecule after the matrix formation [1-5]. Molecularly imprinted matrixes have been developed for over a decade in many fields, such as chromatography, catalysis, artificial antibodies, and sensing devices [6-12].
Until now, the reported general MIP systems have been synthesized with a combination of the monomers which contain the functional groups, the monomer which does not contain the functional group, and the crosslinker. After completing the polymerization, the functional groups in these monomers play an important role that provides the optimum interaction with the template molecule. The crosslinker plays a role that maintains the shape of the residual site after removing the template molecule.
Caffeine (1,3,7-trimethylxanthine) is a natural alkaloid which shows great physiological effects; for example, stimulation of the central nervous system and gastric acid secretion.
The sol-gel process is a method for synthesizing oxidized materials, in which the sol is made by hydrolysis in the liquid phase, which is then changed to a gel. Using the sol-gel process, a product can be produced with a high degree of purity, and a uniformly mixed product can be produced when more than two reactants are used. In addition, the product can be synthesized at comparatively low temperatures using the sol-gel process. Therefore, many types of configurations can be produced, including thin membranes, fibers, and nano-size materials.
Almost all research on MIPs has been conducted using organic monomers because they can be handled more easily than inorganic monomers; therefore there has been little research on MIPs made using inorganic monomers. Until now, almost all of the MIP research with caffeine has also were done using organic polymers [13-16], and little MIP research with caffeine has been conducted using inorganic polymers [17]. Lee etc. [17] reported that an MIP was formed by sol-gel reaction, and the discrimination of caffeine from theophylline (1,3-dimethylxanthine) was conducted using the MIP. In this proess, in order to remove the template molecule, they used calcination, which is known as very complicated process.
In this study, the molecular imprinting was conducted using an inorganic system by sol-gel reaction of silane compounds, and the solvent extraction method was used to remove the template molecule. The effects of the composition of the inorganic polymer, the reaction temperature, and the pH during the synthetic process on the molecular recognition ability of the MIP were estimated.
2. Experimental
2.1. Materials and Instruments
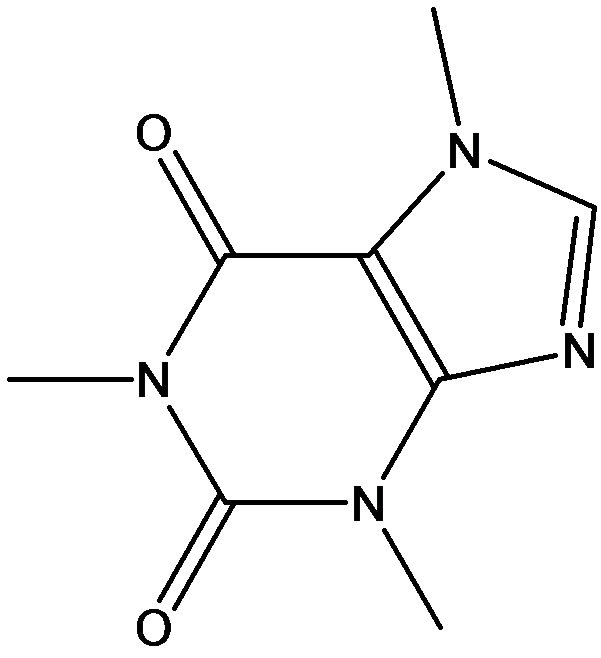
Tetraethoxysilane (TEOS), triethoxymethylsilane (MTES), triethoxyphenylsilane (PTES), caffeine, and theophylline were purchased from Aldrich, and were used without further purification. The chemical structures of the compounds used in this study are shown in Figure 1. Infrared (IR) spectroscopy was performed using a FT-IR680 (Jasco International). High performance liquid chromatography (HPLC) was performed using an ABI, API-3000 HPLC (Agilent) with a flow rate of 1.0 mL/min (mobile phase, water:methanol:acetic acid = 70:27:3, v/v).
2.2. Molecular Imprinting Method of Caffeine by Sol-Gel Process
The most representative synthetic method for the MIP is shown below.
The standard solution of caffeine contained 9.71 g (0.0500 mol) of caffeine dissolved in 1.00 L of water. In a 50 mL Erlenmeyer flask, 0.417 g (2.00 mmol) of TEOS, 0.357 g (2.00 mmol) of MTES, and 1.44 g (5.99 mmol) of PTES were dissolved in 5.0 mL of ethanol with stirring. A 10 mL volume of the caffeine standard solution was added into the Erlenmeyer flask, and this mixture solution was stirred continuously for 10 h. The pH of the solution was controlled to 6.4 - 6.6 using ammonium hydroxide solution. The solution was stirred for 20 min, and then transferred to a 100 mL beaker. The reaction temperature was raised to 50˚C in an oil bath and this solution was kept at 50˚C for 40 h. The formed monolith
 (a)
(a) (b)
(b)
Figure 1. The chemical structure of the compounds used in this study; (a) Caffeine Theophylline; (b) Tetraethoxysilane Triethoxymethylsilane Triethoxyphenylsilane.
was dried under vacuum at 80˚C for 5 h and then ground to obtain a powder with a particle size of 30 - 53 μm, as gathered by sieving. Molecules of caffeine which were used as templates were extracted from the powder using 100 mL of methanol for 30 min. This process was repeated 3 times, and then the powder was dried under vacuum at 80˚C for 3 h. Finally, the MIP was formed.
IR, spectrum (cm−1): -OH, 3300-3700, -phenyl 1650, Si-O-Si 1050-1150.
The nonimprinted polymer (NIP) was prepared under same conditions, but the template was not used.
IR spectrum (cm−1): -OH 3300-3700, -phenyl 1650, Si-O-Si 1050-1150.
2.3. Recognition Ability of Inorganic MIP
The standard solution of both caffeine and theophylline contained 0.100 g of caffeine and 0.100 g of theophylline dissolved in 1.00 L of water. Then, 200 mg of MIP was added to 10 mL of the standard solution in a 100 mL Erlenmeyer flask, and the flask was shaken at 30˚C for 30 min to complete the adsorption process. The solution was then centrifuged at 2000 rpm for 10 min. The above solution was injected into a HPLC to investigate the residual concentrations of caffeine and theophylline, and then the adsorption and selectivity (α) of MIP were determined. The quantity of caffeine adsorbed (Adca) and the quantity of theophylline adsorbed (Adth) were obtained by the difference between the original concentrations and the residual concentrations. The selectivity (α) was obtained by comparing the adsorption of the template (Adca) and analogue (Adth) on MIP. The selectivity was defined as α = Adca/Adth.
3. Results and Discussion
The discriminating ability of the MIP is dependent how well functional groups arrange in the recognition site. To produce a good quality MIP, after the template molecule was removed, the site must accept the returning molecule when we want to reunite both the site and the molecule. The important factors are the position of the functional group, the positions of the hydrophobic part and the hydrophilic part, and the total configuration of the site. The hydroxyl group which remains after the sol-gel reaction could act as a good functional group. In this study, this hydroxyl group is used as a functional group for fabrication of the MIP.
In this study, TEOS, MTES, and PTES were used to synthesize the MIP. Caffeine was used as a template and theophylline, which has a chemical structure similar to that of caffeine, was used as the analogous counterpart compound.
From the results of many preliminary experiments using a 1:1 ratio of TEOS and MTES, we had already found that the optimal reaction temperature was ~50˚C and the optimal pH was 6.4 - 6.6.
While changing the mol ratio of TEOS and MTES, the MIPs were synthesized and the discriminating abilities of these MIPs were estimated; the results are shown in Table 1. The results showed that increasing the mole ratio of MTES produced a decrease in the adsorption amount of both caffeine and theophylline, because the hydrophobic character of the MIP was increased. However, by increasing the mole ratio of MTES, α was increased until TM6. In the case of only using MTES (TM8), α was 2.39, but in the case of only using TEOS (TM1), α was 1.78. Because the α was 1.6 - 1.8 when using nonimprinted polymer (NIP), an α value of 1.78 has almost no meaning; however, an α value of 2.39 of has considerable meaning. In conclusion, the hydrophobic character may be needed to obtain the higher α value. In Table 1, when using the ratio of TEOS:MTES = 1:4 (TM6), the α value was 2.58, which is the highest value in Table 1. The results showed that in the case of only using TEOS (TM1), many hydroxyl groups remained in the MIP, and may help the adsorption of caffeine and theophylline, but too many hydroxyl groups interfered with the discrimination between caffeine and theophylline. The highest α value occurred at TM6, which means that both the hydrophobic character of the MIP and the hydroxyl group are important to obtain the highest α value. In conclusion, the balance of the hydrophobic character and the amount of hydroxyl groups is important to obtain the highest α value. In the next experiment, involving changing the mol ratio of TEOS and PTES, the MIPs were synthesized and the discriminating abilities of these MIPs were estimated; the results are shown in Table 2. The results in Tables 1 and 2 were similar. Increasing the mole ratio of PTES resulted in decreased adsorbed amounts of both caffeine and theophylline. However, increasing the mole ratio of
Table 1. Recognition ability of the inorganic MIP synthesized with TEOS and MTES.

Reaction temperature 50˚C, pH 6.4 - 6.6.
Table 2. Recognition ability of the inorganic MIP synthesized with TEOS and PTES.

Reaction temperature 50˚C, pH 6.4 - 6.6.
PTES, produced an increase in α until PT6. This also means that the hydrophobic character of the MIP is important to achieve the desired discriminating ability. When only PTES was used (TP8) α was 3.09, and this α value was higher than 2.39, which was the α value of TM8 in Table 1. This means that the phenyl group in PTES has more discriminating ability than the methyl group in MTES, because the phenyl group is a larger group than the methyl group. Using PTES, the adsorbed amounts of caffeine and theophylline were decreased comparing to the results using MTES. The larger hydrophobic phenyl group helps produce a greater increase in the α value of MIP, but the adsorbed amounts of caffeine and theophylline showed greater decreases. In Table 2, the highest α value (3.18) was obtained in TP6 and TP7. In TP6, the mole ratio of TEOS:TPES was 1:4, and in TP7, the mole ratio was 1:5. This result also showed that the balance between the hydrophobic character and the amount of hydroxyl groups is important to achieve the highest α value.
Comparing the results of TM1, TM8, TP1, and TP8, the order of the adsorption amounts of caffeine and theophylline is TEOS > MTES > PTES, and the order of the discriminating ability is PTES > MTES > TEOS.
In case of using three compounds (TEOS, MTES, and PTES), we attempted to identify the optimum formulation to produce the highest α value. In Tables 1 and 2, the highest α values were obtained in TM6 (TEOS: MTES = 1:4) and TP6 (TEOS:PTES = 1:4) respectively. To maintain this ratio (1:4) when using three compounds, we changed the ratio only inTEOS: (MTES + PTES) = 1:4. After changing the mol ratios of TEOS, MTES, and PTES, the MIPs were synthesized and the discriminating abilities of these MIPs were estimated; the results are shown in Table 3. A mole ratio of 1:1:3 (TEOS:MTES:PTES) (TMP6) showed the highest α value (3.29) in this study. This result showed that more diverse functional groups
Table 3. Recognition ability of the inorganic MIP synthesized with TEOS, MTES, and PTES.
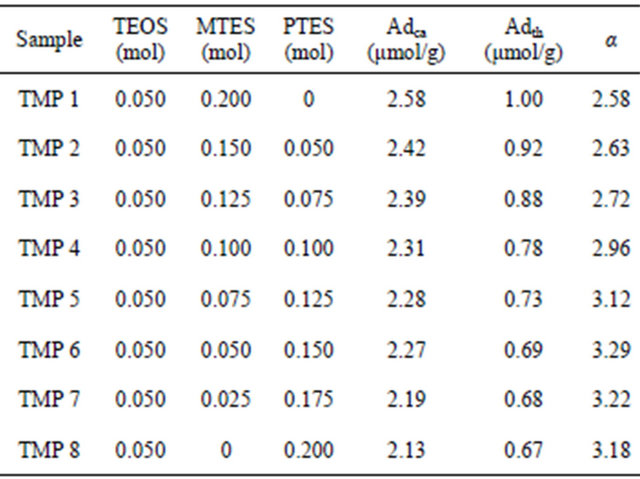
Reaction temperature 50˚C, pH 6.4 - 6.6.
help to increase the α value. The purpose of this study was to mimic a biological compound, such as an enzyme. In an enzymatic system, the binding of the substrate with the enzyme occurs by a very elaborate mechanism. Many functional groups are involved in this mechanism. Therefore, if we use more functionals groups, a higher α value should be obtained. In this study, even though we used only three compounds, it was not easy to determine the optimum formulation. If we use more than four compounds, the optimum formulation could not be obtained by these types of experiments.
In the preliminary experiments using a simple ratio of silanes, the results showed that the optimum reaction temperature was ~50˚C and the optimum pH was 6.4 - 6.6, and these conditions were used in the all of the previous experiments. After identifying the optimal mole ratio, we attempted to check and confirm the optimal temperature to produce the highest α value using this mole ratio, 1:1:3 (TEOS:MTES:PTES). As the reaction temperature was increased by 10˚C from 30˚C to 80˚C, the MIPs were synthesized and the discriminating abilities of these MIPs were estimated; the results are shown in Table 4. The reaction time changed depending on the reaction temperature. At 50˚C (TMPT3) results showed the highest α value in Table 4. We believe that when the reaction is conducted at the different temperature, the number of hydroxyl groups in MIP will be different; therefore we obtained the IR spectra of the sample from TMP1 to TMP6. Even though it is difficult to determine and compare the amount of a functional group using the IR spectrum, by carefully comparing the size of the hydroxyl group peak with the size of other functional group peaks, the comparative size of the IR spectrum peak of the hydroxyl group at the higher temperature was smaller than that at the lower temperature. Also, it has already been reported that the amount of the hydroxyl group is decreased at the higher reaction temperature [18]. In conclusion, the results in Table 4 show that the optimum amount of hydroxyl group is important to achieve the highest α value.
In case of pH, using the simple ratio of the silanes in the preliminary experiments, the results showed that the optimum pH was 6.4 - 6.6. All of the experiments were conducted at pH 6.4 - 6.6. We attempted to check and confirm the optimal pH to achieve the highest α value using the mole ratio, 1:1:3 (TEOS:MTES:PTES). As the pH was increased using ammonium hydroxide solution, the MIPs were synthesized and the discriminating abilities of these MIPs were estimated; the results are shown in Table 5. The results in Table 5 show the highest α value was obtained at pH 6.4 - 6.6. The functional group has a different ionic character at the different pH, and therefore, the interaction of MIP with the substrate molecule is changed at the different pH. The results in Table 5 showed that the interaction of the MIP and the substrate molecule was maximized at pH 6.4 - 6.6.
4. Conclusion
In this study, the MIP was formed by a sol-gel reaction using TEOS, MTES, and PTES. Caffeine was used as a template, and theophylline was used as an analogous
Table 4. Recognition ability of the inorganic MIP synthesized at different temperatures.

TEOS: 0.050 mol; MTES: 0.050 mol; PTES: 0.150 mol; pH 6.4 - 6.6.
Table 5. Recognition ability of the inorganic MIP synthesized at different pH.

TEOS: 0.050 mol; MTES: 0.050 mol; PTES: 0.150 mol; reaction temperature 50˚C.
compound. The discriminating ability of MIP was estimated using these two compounds. The discriminating ability of MIP was dependent of the composition of the MIP. The α value was highest when using the mol ratio (TEOS:MTES:PTES = 1:1:3). Also, the α value was highest when the reaction temperature of the sol-gel reaction was 50˚C, and the pH of the sol-gel reaction was 6.4 - 6.6. In conclusion, the result showed that the balance between the hydrophobic character and the amount of hydroxyl groups in the MIP is important to achieve the highest discriminating ability.
5. Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: 600819)
REFERENCES
- R. Gupta and A. Kumar, “Molecular Imprinting in SolGel Matrix,” Biotechnology Advances, Vol. 26, No. 6, 2008, pp. 533-547.
- A. G. Mayes and K. Mosbach, “Molecularly Imprinted Polymers: Useful Materials for Analytical Chemistry,” Trends in Analytical Chemistry, Vol. 16, 1997, pp. 321- 332.
- S. W. Lee, D. H. Yang and T. Kunitake, “Regioselective Imprinting of Anthracenecarboxylic Acids onto TiO2 Gel Ultrathin Films: An Approach to Thin Film Sensor,” Sensors and Actuators B, Vol. 104, No. 1, 2005, pp. 35-42.
- D. H. Yang, N. Takahara, S. W. Lee and T. Kunitake, “Fabrication of Glucose-Sensitive TiO2 Ultrathin Films by Molecular Imprinting and Selective Detection of Monosaccharides,” Sensors and Actuators B, Vol. 130, No. 1, 2008, pp. 379-385.
- D. H. Yang, Y. R. Ham, M. H. Oh, Y. S. Yoon, J. S. Shin, Y. D. Kim and H. Kim, “Simple Method for the Fabrication of 1-Hydroxypyrene-Imprinted TiO2 Gel Nanofilms,” Current Applied Physics, Vol. 9, No. 2, 2009, pp. e136- e139. doi:10.1016/j.cap.2008.12.043
- K. Haupt and K. Mosbach, “Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors,” Chemical Review, Vol. 100, No. 7, 2000, pp. 2495-2504.
- H. W. Sun, F. X. Qiao and G. Y.Liu, “Characteristic of Theophylline Imprinted Monolithic Column and Its Application for Determination of Xanthine Derivatives Caffeine and Theophylline in Green Tea,” Journal of Chromatography A, Vol. 1134, No. 1-2, 2006, pp. 194-200.
- J. Yin, G. Yang and Y. Chen, “Rapid and Efficient Chiral Separation of Nateglinide and Its L-Enantiomer on Monolithic Molecularly Imprinted Polymers,” Journal of Chromatography A, Vol. 1090, No. 1, 2005, pp. 68-75.
- P. D. Martin, G. R. Jones, F.Stringer and I. D.Wilson, “Comparison of Normal and Reversed-Phase Solid Phase Extraction Methods for Extraction of β-Blockers from Plasma Using Molecularly Imprinted Polymers,” Analyst, Vol. 128, No. 4, 2003, pp. 345-350.
- O. Bruggemann, A. Visnjevski, R. Burch and P. Patel, “Selective Extraction of Antioxidants with Molecularly Imprinted Polymers,” Analytica Chimica Acta, Vol. 504, No. 1, 2004, pp. 81-88.
- H. Yan and K. H. Row, “Molecularly Imprinted Monolithic Stationary Phases for Liquid Chromatographic Separation of Tryptophan and N-CBZ-Phenylalanine Enantiomers,” Biotechnology and Bioprocess Engineering, Vol. 11, 2006, pp. 357-363.
- D. Cunliffe, A. Kirby and C. Alexander, “Molecularly Imprinted Drug Delivery Systems,” Advanced Drug Delivery Reviews,” Vol. 57, No. 12, 2005, pp. 1836-1853.
- L. Tangbin, T. Xin and L. Songjun, “Selective Adsorption and Recognition by Molecularly Imprinted Polymer: A Study on Molecular Self-Assembly and Its Effect on Selectivity,” Polymer-Plastics Technology and Engineering, Vol. 46, No. 6, 2007, pp. 613-619.
- S. L. Kyung, S. K. Dae and S. K. Beom, “Biodegradable Molecularly Imprinted Polymers Based on Poly(ε-Caprolactone),” Biotechnology and Bioprocess Enginering, Vol. 12, No. 2, 2007, pp. 152-156.
- F. Keith, M. Edmond and R. Fiona, “Predicting the Performance of Molecularly Imprinted Polymers: Selective Extraction of Caffeine by Molecularly Imprinted Solid Phase Extraction,” Analytica Chimica Acta, Vol. 566, No. 1, 2006, pp. 60-68.
- Y. Hongyuan, J. Longmei and H. R. Kyung, “Special Selectivity of Molecularly Imprinted Monolithic Stationary Phase,” Journal of Liquid Chromatography and Related Technologies, Vol. 28, No. 20, 2005, pp. 3147- 3155.
- S. C. Lee, H. M. Lin and H. Chen, “Studies on the Preparation and Properties of Inorganic Molecularly Imprinted Polymer (MIP) Based on Tetraethoxysilane and Silane Coupling Agents,” Journal of Applied Polymer Science, Vol. 114, No. 6, 2009, pp. 3994-3999.
- Y. J. Shin, M. H. Oh, Y. S. Yoon and J. S. Shin, “Hard Coatings on Polycarbonate Plate by Sol-Gel Reactions of Melamine Derivative, PHEMA, and Silicates,” Polymer Engineering and Science, Vol. 48, No. 7, 2008, pp. 1289- 1295.
NOTES
*Corresponding author.

