Journal of Electronics Cooling and Thermal Control
Vol. 3 No. 1 (2013) , Article ID: 29383 , 6 pages DOI:10.4236/jectc.2013.31001
A Study of Water Supercooling
1Institute for Computational Engineering and Science, University of Texas at Austin, Austin, USA
2Department of Mechanical Engineering, Amirkabir University of Technology, Tehran, Iran
Email: gholaminejad@utexas.edu, hoseinir@aut.ac.ir
Received January 16, 2013; revised February 18, 2013; accepted February 27, 2013
Keywords: Supercooling; Freezing; Nucleation; Phase Change; Mpemba Effect
ABSTRACT
The objective of this paper is to investigate water supercooling. Supercooling occurs when a liquid does not freeze although its temperature is below its freezing point. In general, supercooling is an unstable condition and occurs under special conditions. The parameters that influence supercooling stability and probability of occurrence include freezer temperature and water’s initial temperature. In this paper, it is shown that with a freezer temperature range of −3˚C to −8˚C, supercooling is most likely to happen and is independent of the water’s initial temperature. Furthermore, as the freezer temperature decreases, the probability of nucleation increases, causing instant freezing. Finally, it is concluded that the Mpemba effect, in which initially hot water freezes faster than initially cold water, is due to the supercooling instability in initially hot water in which nucleation agents are more active.
1. Introduction
By observing the water’s phase diagram, it seems impossible to have liquid water several degrees below its freezing point. When water temperature reaches 0˚C, (at atmospheric pressure) its temperature remains constant and phase transition process occurs. However, there are circumstances in which water temperature drops below its freezing point, but no phase transition happens while water remains in liquid phase. This condition is called supercooling.
Supercooling phenomenon is of particular interest in food industry. While in transport, foods like fruits and vegetables require storage in a cold environment where the temperature is several degrees below freezing point. However, freezing the food would reduce its quality by damaging food cells and changing its color. However, if one can store food in a subfreezing temperature without freezing, there would be no loss of quality. For instance, unpeeled garlic was stored in −6˚C for a week, without freezing [1]. Moreover, supercooling is necessary for the survival of some creatures and plants in nature. When an organism’s fluid is cooled below its freezing point, it is probable that ice nucleation happens inside its tissues, which causes lethal freezing. To survive, the specie must either tolerate this freezing or somehow prevent nucleation. For instance, insects are seen to be supercooled to extremely low temperatures of −40˚C. Fish also use the supercooling phenomenon to survive in subfreezing temperatures. The Polar Teleost fish is a good example of a species that supercools to −1.9˚C while its body fluid’s freezing point is −0.6˚C [2].
Supercooling was first introduced by Brown in 1916 to explain why hot water pipes burst more often than cold water pipes [3]. He concluded that boiled water is more likely to experience supercooling than non-boiled water, and related this to the different amount of dissolved gases in hot and cold water pipes. Also, he observed that when supercooled water was frozen, its ice structure was clear and without air bubbles. 32 years later, Dorsey reported that there is no connection between dissolved gases and supercooling, but he agreed with Brown that boiled water is more likely to experience supercooling than non-boiled water [4]. He concluded that this is because heating deactivates nucleation sites in the water.
In 1955, Mossop investigated freezing of supercooled water of high purity. In an experiment, water was supercooled down to −34.5˚C [5]. He discovered that nucleation happens when supercooled water is exposed to air. This was related to freezing nuclei found in the air.
In 1977, Gilpin carried out numerous experiments on water supercooling and confirmed that hot tap water supercools more than cold water [6]. Also, he reported that hot water in an open container is less probable to experience supercooling, due to the fact that it can absorb impurities from air.
In 1995, Auerbach carried out extensive experiments in which he varied freezer temperature and water’s initial temperature before placing it in the freezer [7]. He observed that hot water is likely to nucleate in higher temperatures compared to cold water, which tends to nucleate in lower temperatures. However, due to limited number of experimental runs, he could not confirm whether hot water nucleates sooner or not.
Despite many experimental studies on water supercooling, there is still uncertainty on the parameters that influence this phenomenon. The objective of the present study is to first confirm the existence of this phenomenon and then study the parameters influencing its occurrence and stability. Finally, we have explained how Mpemba phenomenon is related to supercooling.
2. Freezing of Water, Definitions
Melting Point (MP)/Freezing Point (FP): Melting point of a solid is the temperature at which all solid crystals have disappeared while being heated slowly. Since it was believed that a solution would freeze when cooled to its MP, the freezing point of a solution was thought to be the same as its MP. However, there are a number of situations in which a solution does not freeze at its freezing point. For a solution to freeze, not only should the liquid’s temperature reach FP, but also there should be nucleation sites to help the molecular structure change from liquid to solid. If any of these are absent freezing will not occur [8].
Nucleation Temperature (NT): Nucleation temperature is the temperature at which the first ice crystals appear in a solution. It is also referred to as supercooling point (SCP) or crystallization temperature [8]. Nucleation is accompanied by an increase in solution temperature, due to the release of the latent heat of crystallization [9].
Nucleation Agent: In order for water to freeze, its molecular structure needs to change from liquid into ice. This change is possible only if water molecules find nucleation sites. Aggregates found in water or dissolved gasses in it can serve as these nucleation sites, which are referred to as nucleation agents.
Homogenous/Heterogeneous Nucleation: There are two types of nucleation: homogeneous and heterogeneous. Nucleation caused by electrostatic attraction between water polar molecules is referred to as homogenous nucleation. Since such attractions are weak, a large number of molecules need to be present to initiate nucleation. If nucleation happens with the aid of an extrinsic nucleator, it is referred to as heterogeneous nucleation [8]. An example of an extrinsic nucleator is frost or ice. If ice is dropped into supercooled water, nucleation occurs instantly.
Supercooling Capacity: Supercooling capacity is the difference between MP temperature and NT. It shows how much a solution has been supercooled.
3. Experimental Setup
3.1. Freezing Compartment
To study water freezing, one needs to possess a cold chamber. Some researchers have used cold air in a commercial freezer. A major problem with this mechanism is that heat transfer rate from the sides of the water beaker (convection cooling) is different from its bottom (conduction heat transfer), which can affect supercooling.
In the present study, a cold liquid bath is used as the freezing compartment in order to solve the above problem. There were 30 liters of ethylene glycol (liquid) in the bath, which was cooled by evaporator coils of a vapor/compression refrigerator. To have an isotherm cold liquid, a circulator was placed in the ethylene glycol (Figure 1).
Since small distractions including compressor vibration would influence water supercooling, the compressor was turned off during the test period. However, the bath temperature did not change considerably due to the fact that the test period was short (about 10 minutes) and the size of bath was large (30 liters of ethylene glycol).
3.2. Preparation of Samples
To study the effect of water’s initial temperature on supercooling, water with different initial temperatures was to be prepared. The initial temperature of water is defined as the temperature of water at the start of the experiment before placing it into the freezer. The heating mechanism must be such that other parameters of water, such as dissolved gases, remain almost constant for different initial temperatures to minimize their influence on supercooling. If the samples were heated directly to the desired temperature, then the amount of dissolved gases, specifically CO2, would change. The amount of dissolved gases is believed to influence water supercooling [7,10].
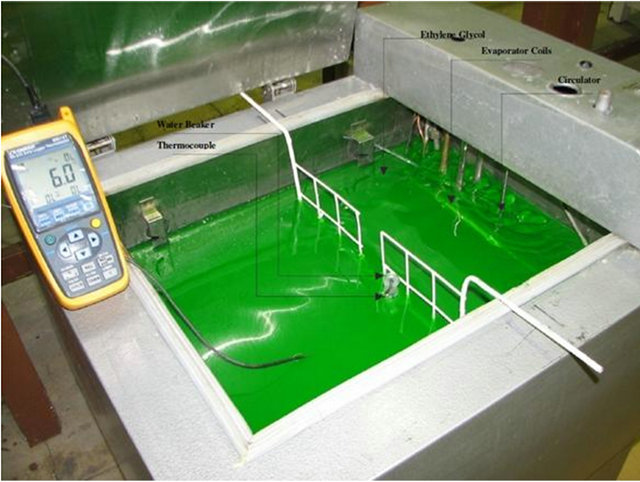
Figure 1. Cold bath with ethylene glycol as the freezing media.
To overcome this problem, water was first heated to 95˚C, out of which 50 ml was cooled in the ambient temperature of 22˚C in a beaker. When water temperature reached the desired value, the beaker was placed in the freezer.
Next, the beaker containing water was drowned in the freezer such that its top surface was out of ethylene glycol making it lose heat homogenously (Figure 1).
An Omega HH147 data logger was used to record water temperature. An Omega5SRTC K type thermocouple was immersed in water and connected to the data logger.
Distilled water was used so that dissolved materials would not influence water supercooling. Each test was performed with 50 ml of distilled water in a beaker. Moreover, the experiments were conducted in an environment with an ambient temperature of 22˚C and a pressure of 663 mmHg. The experiments were performed with initial water temperature in the range of 20˚C to 90˚C and freezer temperature range of −4˚C to −12˚C.
4. Results
4.1. Water Supercooling
The first objective of the experiments was to see whether supercooling occurs or not. In one of the experiments 500 ml of distilled water with initial temperature of 65˚C was placed in a freezer temperature of −8˚C. Water temperature “History” is shown in Figure 2. As one can see when water temperature reaches 0˚C its temperature does not remain constant and phase change does not occur. Instead, supercooling occurs and water temperature drops below 0˚C. After about 25 seconds water temperature reaches −7˚C while it is still in liquid phase. If one leaves the water in this state without any disturbance, supercooling would sustain for hours. In one of the present experiments liquid water was in supercooling state for more than 5 hours.
To illustrate how nucleation can end supercooling state and initiate phase change, 1 ml of frost is added to the supercooled water in the previous experiment, Figure 3(a). Moments after the frost is added, nucleation starts and water begins to freeze (Figure 3(b)). After 2.53 seconds, thermometer shows 7˚C rise in water temperature (Figure 3(c)). This rise in temperature is due to the release of latent heat of crystallization. Finally, 3.13 seconds after the addition of frost, most of the water is frozen while its temperature is 0˚C (Figure 3(d)). At the end, water is a mixture of ice and liquid water at 0˚C.
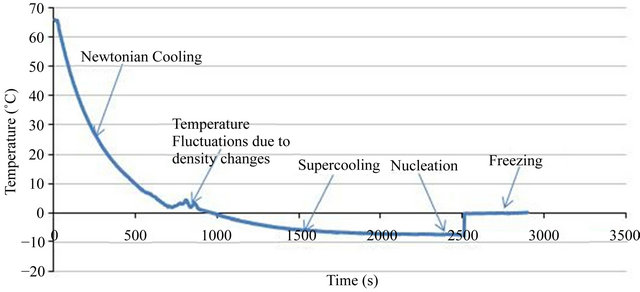
Figure 2. Water temperature graph of frost addition experiment.
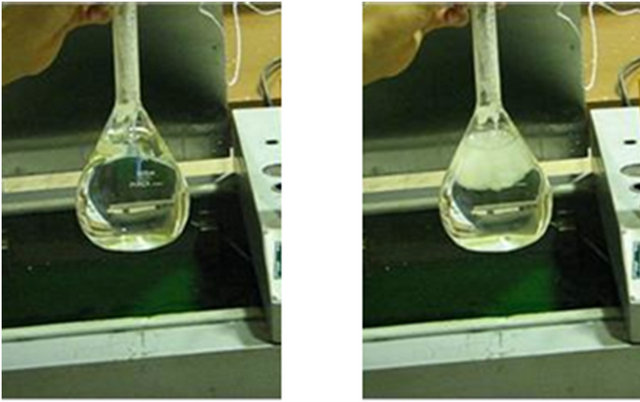
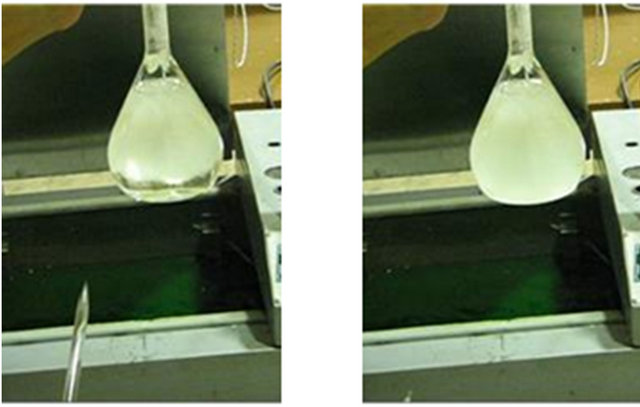
(a) (b) (c) (d)
Figure 3. (a) Water nucleation forced by pouring frost at t = 0; (b) 1.13 seconds after nucleation; (c) 2.53 seconds after nucleation; (d) 3.13 seconds after nucleation.
Five different regions can be identified in Figure 2:
1): Cooling is Newtonian [7].
2): Temperature fluctuations are seen in this region. This is primarily due to density changes near 4˚C, which would cause side wall boundary layer to collapse [11].
3): At 0˚C the phase change process does not happen and water supercools until its temperature reaches −7˚C.
4): Nucleation is forced by dropping some frost in water beaker. Due to release of the latent heat of crystallization, water temperature rises.
5): Water becomes a mixture of ice and liquid.
Figure 3 shows how nucleation occurred when frost was added to the supercooled water. It should be noted that supercooling did not occur for any combination of water initial temperature and freezer temperature. Therefore, we conducted experiments to study the effect of these two parameters.
4.2. Freezer Temperature and Water’s Initial Temperature
Freezer temperature was varied in the range of −4˚C to −12˚C to study its effect on supercooling. The results are shown in Table 1. The results are as follows:
- For freezer temperature range of −4˚C, −6˚C and −8˚C, nucleation was not observed and water remained in supercooled condition for as long as five hours in one of the tests. This implies that there were no nucleation agents present to initiate freezing.
- For freezer temperature of −10˚C nucleation was random, but it was higher in the initially hot water, indicating higher activity of nucleation agents in initially hot water.
- For freezer temperature of −12˚C nucleation always happened and supercooling was rarely seen, indicating
Table 1. Supercooling of water at different freezer temperatures and different water initial temperatures. Freezer temperature shows the cooling bath temperature, water initial temperature is the initial temperature of water at the time it was put in the cooling bath, freezing shows whether the water froze or remained in the supercooling state (that is below freezing point without freezing because of lack of nucleation sites), and nucleation temperature shows the temperature at which water lost its supercooling state and froze (Dash lines correspond to runs in which water stayed in supercooling state without freezing). The results show that in general by increasing water initial temperature and decreasing cooling bath temperature, water tends to lose its supercooling state sooner.

that nucleation agents were active in both initially hot water and initially cold water.
Therefore, it can be deduced that in general, initially hot water has lower supercooling capacity than initially cold water. This behavior is also observed in a study of strawberry supercooling [9].
4.3. Analysis and Discussion
As mentioned before, for freezer temperature range of −4˚C to −8˚C supercooling occurs for a long time and is independent of the water’s initial temperature. This is due to the lack of nucleation sites, as discussed before. For colder freezer temperatures such as −10˚C and −12˚C, supercooling becomes unstable and nucleation happens. At −10˚C, the nucleation agents are more active in initially hot water than initially cold water. But at a freezer temperature of −12˚C, they are so active that they cause nucleation in both hot and cold water. Therefore, it can be deduced that in general, nucleation sites increase as freezer temperature is decreased and initial temperature of water is increased.
There are several possibilities to explain this behavior, specifically the difference between supercooling of initially hot water and initially cold water. Some believe that water’s cooling history play a role, specifically dissolved gasses [12,13]. Initially hot water has less dissolved gasses compared to initially cold water. However, this cannot explain this phenomenon since less dissolved gasses implies less nucleation sites. An acceptable explanation of this behavior is yet to be found.
5. Supercooling and the Mpemba Effect
The fact that in some specific conditions initially hot water freezes faster than initially cold water is referred to as the Mpemba effect. This phenomenon was first introduced to modern science in 1969 by Erasto B. Mpemba, a Tanzanian high school student [14]. This seems counter-intuitive at first, since initially hot water has higher internal energy than initially colder water, and as a result of Newton’s law of cooling initially hot water should take longer to freeze [10].
Many theories have been proposed to explain this phenomenon. In 1969, Kell suggested that surface evaporation could explain the Mpemba effect [15]. Initially hot water has higher surface evaporation and hence would lose more mass and energy compared to initially cold water. However, later studies showed that this reduction in mass and energy is insufficient to make initially hot water to freeze faster than initially cold water [12,14].
In 1971, Deeson suggested that convection currents cause initially hot water to have a higher rate of heat transfer from its top surface [16]. In the cooling process, water does not have a uniform temperature distribution. The hot layers travel to the surface due to natural convection, causing the surface temperature to be higher than the average temperature of water. He proposed that when initially hot water’s average temperature reaches initially cold water temperature, the initially hot water would have a higher cooling rate due to the higher surface temperature, and therefore, would freeze faster.
Dissolved gases in water is also believed to have a role in the Mpemba effect, since initially hot water has less dissolved gases than initially cold water [12,13,17]. However, Auerbach reported that dissolved gases in water do not influence Mpemba effect [7]. This was proven by varying the amount of dissolved gases in both initially hot and cold water as mentioned before.
If these theories are correct, they are still unable to explain the experiments in which initially hot water freezes much faster than initially cold water. In one of our experiments, initially cold water froze five hours after initially hot water froze. Noting that because initially hot water tends to nucleate sooner than initially cold water, suggests that supercooling instability of initially hot water plays an important role in this phenomenon. Specifically, when the nucleation starts in initially hot water, the initially cold water is still in the supercooling phase. The visual examination of the supercooled water may give the wrong impression that its temperature is above 0˚C since water is still in the liquid phase while the initially hot water appears completely frozen. This may result in a conclusion that initially hot water freezes faster than initially cold water. Figure 4" target="_self"> Figure 4 shows a case in which Mpemba phenomenon occurs due to supercooling. This experiment was done with a bath temperature of −10˚C. Both initially hot/cold water experienced supercooling. However, the supercooling state of initially hot water is not stable and after seconds of passing the freezing point, nucleation occurs and its temperature jumps to the freezing point. However, this does not happen in initially cold water. The supercooling state of initially cold water remains stable for 30 minutes and phase transition does not occur. At this stage, initially hot water is completely frozen. Moreover, visual examination of cold water may cause confusion that it is still above 0˚C, because it is still in liquid phase, while in fact it is being supercooled. After 30 minutes of supercooling, nucleation starts in initially cold water and its temperature jumps to 0˚C. This jump is due to release of the latent heat of crystallization. At this time, initially cold water is a mixture of ice and water and the freezing process continues to turn the remaining water into ice.
6. Conclusions
Water supercooling was studied by varying water’s initial temperature and freezer temperature through numerous
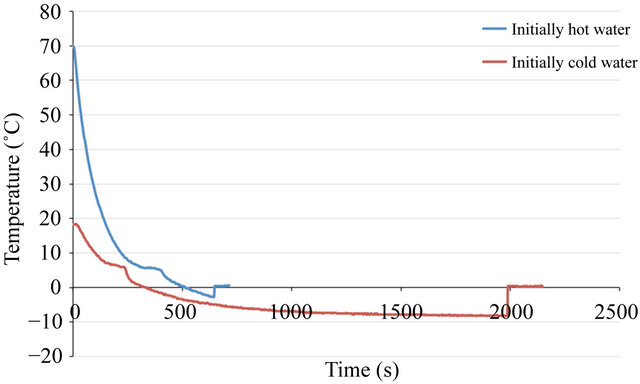
Figure 4. Temperature history of an experiment in which Mpemba Effect was observed.
experiments. It is concluded that supercooling is unstable at low freezer temperatures. Moreover, it is observed that initially hot water tends to nucleate and freeze sooner compared to initially cold water.
Furthermore, it is concluded that the Mpemba effect, in which initially hot water freezes faster than initially cold water, is due to the supercooling instability in initially hot water. Specifically, initially hot water nucleates faster than initially cold water and as a result, starts to freeze, while the initially cold water is still in the supercooled liquid phase.
7. Acknowledgements
We are in debt to Mohammad Samadi who helped us in conducting the experiments. We have to specially thank Behnood Gholami who proofread the manuscript and gave us valuable feedback. All of the experiments have been performed in Thermodynamics Lab of Mechanical Engineering Department of Amirkabir University of Technology.
REFERENCES
- C. James, V. Seignemartin and S. J. James, “The Freezing and Supercooling of Garlic (Allium sativum L.),” International Journal of Refrigeration, Vol. 32, No. 2, 2009, pp. 253-260. doi:10.1016/j.ijrefrig.2008.05.012,
- A. DeVries, “Biological Antifreeze Agents in Coldwater Fishes,” Comparative Biochemistry and Physiology Part A: Physiology, Vol. 73, No. 4, 1982, pp. 627-640. doi:10.1016/0300-9629(82)90270-5,
- F. Brown, “The Frequent Bursting of Hot Water Pipes in Household Plumbing Systems,” Physical Review, Vol. 8, No. 5, 1916, pp. 500-503. doi:10.1103/PhysRev.8.500
- N. Dorsey, “The Freezing of Supercooled Water,” Transactions of the American Philosophical Society, Vol. 38, No. 3, 1948, pp. 246-328. doi:10.2307/1005602
- S. Mossop, “The Freezing of Supercooled Water,” Proceedings of the Physical Society. Section B, Vol. 68, No. 4, 1955, pp. 193-208. doi:10.1088/0370-1301/68/4/301
- R. Gilpin, “The Effects of Dendritic Ice Formation in Water Pipes,” International Journal of Heat and Mass Transfer, Vol. 20, No. 6, 1977, pp. 693-699. doi:10.1016/0017-9310(77)90057-6,
- D. Auerbach, “Supercooling and the Mpemba Effect: When Hot Water Freezes Quicker than Cold,” American Journal of Physics, Vol. 63, No. 10, 1995, pp. 882-885. doi:10.1119/1.18059
- K. Zachariassen and E. Kristiansen, “Ice Nucleation and Antinucleation in Nature,” Cryobiology, Vol. 41, No. 4, 2000, pp. 257-279.
- R. Martins and V. Lopes, “Modelling Supercooling in Frozen Strawberries: Experimental Analysis, Cellular Automation and Inverse Problem Methodology,” Journal of Food Engineering, Vol. 80, No. 1, 2007, pp. 126-141. doi:10.1016/j.jfoodeng.2006.05.009
- M. Jeng, “The Mpemba Effect: When Can Hot Water Freezes Faster than Cold?” American Journal of Physics, Vol. 74, No. 6, 2006, pp. 514-523. doi:10.1119/1.2186331
- I. Firth, “Cooler,” Physics Education, Vol. 6, No. 1, 1971, pp. 32-41. doi:10.1088/0031-9120/6/1/310
- M. Freeman, “Cooler Still—An Answer?” Physics Education, Vol. 14, No. 7, 1979, pp. 417-421. doi:10.1088/0031-9120/14/7/314
- B. Wojciechowski, I. Owczarek and G. Bednarz, “Freezing of Aqueous Solutions Containing Gases,” Crystal Research Technology, Vol. 23, No. 7, 1988, pp. 843-848.
- E. Mpemba and D. Osborne, “Cool?” Physics Education, Vol. 4, No. 3, 1969, pp. 172-175. doi:10.1088/0031-9120/4/3/312
- G. Kell, “The Freezing of Hot and Cold Water,” American Journal of Physics, Vol. 37, No. 5, 1969, pp. 564-565. doi:10.1119/1.1975687
- E. Deeson, “Cooler-Lower Down,” American Journal of Physics, Vol. 6, No. , 1971, pp. 42-44. doi:10.1088/0031-9120/6/1/311
- R. Pearce, “Plant Freezing and Damage,” Annals of Botany, Vol. 87, No. 4, 2001, pp. 417-424.

