Advances in Materials Physics and Chemistry
Vol.06 No.09(2016), Article ID:70393,13 pages
10.4236/ampc.2016.69025
Counterion Binding in Aqueous Solutions of Poly(vinylpyridines) as Assessed by Potentiometric Titration
Jim D. Roach*, Mandy M. Bondaruk, Abdulaziz Al-Abdulghani, Zaid Shahrori
Pre-Medical Education Unit, Weill Cornell Medicine-Qatar, Doha, Qatar

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 18, 2016; Accepted: September 3, 2016; Published: September 6, 2016
ABSTRACT
The extent to which counterions bind to polyelectrolytes influences a variety of polymer-based applications, including polyelectrolyte enhanced ultrafiltration and forward osmosis using polyelectrolytes as draw agents. Potentiometric titrations of poly (2-vinylpyridine) (P2VP), poly (3-vinylpyridine) (P3VP), and poly (4-vinylpydine) (P4VP) were performed using HBr, HCl, HNO3, and HClO4 in both the presence and absence of added NaCl. Because of the systematic differences among the three polyelectrolytes, titration results provide insight into the role of polymer structure in the relative extents to which various counterions bind. Titration data reveal that ionization properties vary as functions of polymer investigated, titrant used, degree of protonation, and added salt concentration. Acid dissociation constants of the pyridinium moieties were found to generally increase with increasing degree of protonation, though appreciable differences were exhibited among the three polymers investigated. For all three polymers, Cl− demonstrated the lowest affinity for the charged pyridinium residues, while the affinities associated with Br− and 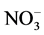 were nearly identical to each other. The relative extent of binding for
were nearly identical to each other. The relative extent of binding for 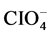 varied across the polymers investigated, and was greatest for P4VP.
varied across the polymers investigated, and was greatest for P4VP.
Keywords:
Poly(vinylpyridine), Counterion Binding, Potentiometric Titration, Perchlorate

1. Introduction
Aqueous solutions of responsive polymers, the properties of which change according to bulk conditions, are used in a variety of colloid-based processes [1] - [4] . Of particular applicability in these processes are pH-responsive polymers that contain acidic [5] or basic [6] moieties. Common polyacids include polyacrylic acid and polymethacrylic acid, while polybases include polyvinyl amine and polyethylenimine. These polymers are used in a variety of applications including drug delivery, wastewater remediation, adhesion, lubrication, and colloid stabilization. Because properties like solution density, viscosity, polymer charge density, counterion binding, and polymeric conformation are greatly influenced by the degree to which pH-responsive polymers are protonated, their ionic equilibria are of immense interest.
The polybases poly(2-vinylpyridine) (P2VP), poly(3-vinylpyridine) (P3VP), and poly (4-vinylpyridine) (P4VP) (Figure 1), have been investigated for use in wide-ranging applications. The structures of these polyelectrolytes impart exploitable properties to materials, like membranes and gels, made from them. Investigations exploring the uses of PVP include the stabilization of non-aqueous emulsions [7] and [8] by P2VP; stimuli-responsive nanoparticles, nanogels, and capsules made using P3VP [9] ; the action of colloidal silica films on nano-composites [10] , the removal of perchlorate from aqueous solution [11] , protonation and diffusion phenomena in weak anion-exchange membranes [12] , the encapsulation of multi-walled carbon nanotubes [13] , the preparation of micro porous membranes [14] , and the isolation of free amino acids [15] using P4VP. The results of many of these investigations demonstrate the importance that the degree of protonation has in influencing the aqueous solution properties of PVP; properties like viscosity, swelling, and especially counterion binding.
The concept of condensation of counterions around a charged polymer was introduced by Oossawa in 1957 [16] and later expanded by Manning [17] . In his simple two- state model, Oosawa considered counterions to be either “bound” to the polyion or “unbound” in bulk aqueous solution. Affecting the extent to which counterions bind is the electrical potential of the polyion. Equation (1) represents Oosawa’s relation between the apparent degree of polyion dissociation (β), the apparent volume fraction in which bound counterions are located (φ), and a dimensionless quantity describing the electrical potential of the polyion (P).
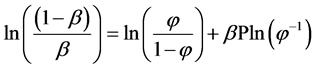 (1)
(1)
The electrical potential parameter is greatly influenced by the spacing of charged
Figure 1. Structures of poly(vinylpyridines) investigated.
moieties along the polymer and its degree of protonation. With the spacing between charged sites increasing in the order P2VP < P3VP < P4VP, the titration behavior of the three PVPs provides insight into the influence of P and degree of protonation on counterion condensation.
Several studies have investigated the degree of protonation and other solution properties of the PVPs, beginning with Fuoss and Strauss [18] . Kirsh et al. [19] investigated P2VP and P4VP in alcohol-water solutions via potentiometric titration with HCl. Satoh et al. [20] titrated P2VP and P4VP with HCl and benzenesulfonic acid to assess the influence of hydrophobic interactions on counterion binding. Yoshida [21] investigated the potentiometric titration properties of P4VP with HCl as a function of polymer molecular weight, observing that it did not influence ionization in the range 13.4 kDa to 223 kDa. Roach et al. [11] titrated P4VP with HCl and HClO4 in the presence and absence of added NaCl to assess the effect of ionic strength on the apparent polymer dissociation constant. Because the PVPs are water-soluble only at a sufficient degree of protonation, pH adjustment provides a mechanism whereby polymer can be recovered for reuse in applicable colloid-based processes, making PVPs potentially desirable in these applications.
In some colloid-based applications, like polyelectrolyte-enhanced ultra filtration (PEUF) processes, a higher degree of counterion binding is desired [22] ; in other applications, like desalination by forward osmosis (FO) where polyelectrolytes or charged nanoparticles are used as draw agents, a lesser degree of counterion condensation leads to higher draw solution ionic strengths and greater water flux values [23] . In this study, potentiometric titrations of aqueous solutions of P2VP, P3VP, and P4VP were performed using HBr, HCl, HNO3, and HClO4. The apparent polymer dissociation constants were determined as functions of degree of protonation for each of the strong acids used. These data were then used to assess the relative extents to which the various counterions bind to the respective polymers.
2. Materials and Methods
2.1. Chemicals
Poly(2-vinylpyridine) with an average molecular weight of 40,000 Da and poly (4-viny- lpyridine) with an average molecular weight of 50,000 Da were obtained from Polyscience Inc. Poly(3-vinylpyridine) with an average molecular weight of 50,000 Da was obtained from Apollo Scientific Limited. The molar concentrations of polymer solutions are reported in terms of their respective monomeric units. Solutions were prepared using reagent grade sodium chloride, hydrobromic acid, hydrochloric acid, nitric acid, perchloric acid, potassium hydrogen phthalate, and sodium hydroxide.
Polymer solutions were prepared by placing approximately 0.26 g of PVP in a 60 mL bottle along with an aliquot of specified strong acid, a 12 mm stir bar, and enough water to fill the bottle about two-thirds. The sealed mixture was stirred upright for several hours and then inverted for additional stirring until full dissolution was achieved. Finally, the contents were washed into a 100 mL volumetric flask. This manner of preparation avoids clumping of polymer on the vessel walls and hastens the dissolution process.
2.2. Potentiometric Titrations
Solution pH values were determined using a Thermo Orion model 3 Star pH meter equipped with a 9102BNWP electrode calibrated using Gran plots created by the software GLEE [24] . The calibration provided accurate readings in the pH range 2.0 - 5.0. For all potentiometric titrations, the concentration of polymeric pyridine residue was approximately 0.02 M and the initial volume of polymer solution was 30.0 mL. Standardized strong acid titrants were approximately 0.1 M in concentration. Aqueous polymer solutions required an initial partial protonation of between 30% and 55%, depending the polymer and counter-anion being investigated. A Grant Instruments Ltd. model GP200 constant temperature water bath maintained the solutions being titrated at 25.0˚C ± 0.1˚C throughout all experiments. Titrations were performed in triplicate and average pH values were used in calculating the apparent acid dissociation constants, pKa,app. Standard deviations from the triplicate readings were small, equating to < 0.02 pH unit.
The acid dissociation constant Ka,app for protonated PVP can be expressed as in Equation (2),
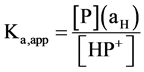 (2)
(2)
where [P] and [HP+] represent the concentrations of deprotonated and protonated pyridine residues in solution, respectively, and aH is the activity of bulk proton. Taking −log10 of both sides provides Equation (3).
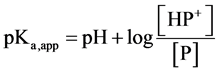 (3)
(3)
The total concentration of pyridine residue in solution, Ptot, is defined by Equation (4). The degree of
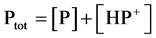 (4)
(4)
protonation at a given pH value, a, was determined as the difference between the moles of strong acid added, nHX, and that required to achieve the same pH in a blank sample at the same approximate ionic strength, nblank, using Equation (5).
 (5)
(5)
Ionic strength values, I, were calculated assuming no polymeric contribution. All activity coefficients, 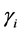 , needed to relate measured activities to concentrations were calculated using the Davies equation (see Equation (6)).
, needed to relate measured activities to concentrations were calculated using the Davies equation (see Equation (6)).
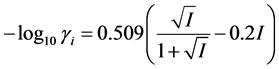 (6)
(6)
Combining and rearrangement of Equations (3)?(5) provides Equation (7).
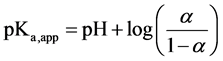 (7)
(7)
Variations of pKa,app with a have been used previously to infer polymeric conformational transitions [14] and to evaluate changes in thermodynamic parameters associated with protonation [25] .
3. Results and Discussion
3.1. Polymer Dissolution
As mentioned previously, appreciable protonation of the polymers was required to achieve dissolution in water. The minimum protonation needed varied as a function of polymer and strong acid used, but was generally in the range of 30% - 55%. The range is in agreement with that reported by Satoh et al. [20] for P2VP and P4VP using HCl; by Mika and Childs [14] for P4VP using HCl; and by Roach et al. [11] for P4VP using HCl and HClO4. This variation is attributable to the extent to which the counter-anions bind to cationic sites on the polymeric strand. A greater level of binding requires a higher degree of protonation to achieve dissolution. From each titration performed, the pKa,app at a = 0.55, pKa,app(0.55), was determined and used to compare behavior across the polymers and acids studied; 55% protonation is the lower limit of solubility for P3VP and P4VP with  counterions. A similar technique was employed previously by Yoshida [21] when assessing the aqueous solution properties of P4VP with HCl.
counterions. A similar technique was employed previously by Yoshida [21] when assessing the aqueous solution properties of P4VP with HCl.
3.2. Polymer Titrations in the Absence of Added Salt
The dependences of pKa,app on a for P2VP, P3VP, and P4VP, in the absence of added salt, are depicted in Figures 2-4. For all three polymers with all four strong acids studied,
Figure 2. Titrations of P2VP at 25˚C with no added salt.
Figure 3. Titrations of P3VP at 25˚C with no added salt.
Figure 4. Titrations of P4VP at 25˚C with no added salt.
pKa,app is observed to decrease with increasing a in the range a = 0.4 - 0.8. This trend is attributable to the increasingly cationic character of the solvated polymer at higher a values. Protonated pyridine residues repulse hydronium, thus hindering their acquisition by neighboring residues and increasing the observed acid dissociation constant. While the general trend is deceasing pKa,app with increasing a, the slopes vary across the three polymers investigated. This variation can be evaluated by incorporation of a factor, q, into an expression similar to Equation (7), except pKa is now the intercept of pH
vs.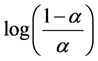 .
.
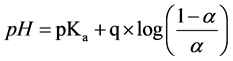 (8)
(8)
Values for parameter q were obtained from plots of pH vs. 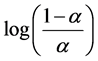 and are
and are
summarized in Table 1. Parameter q is generally greater than 1 and is observed to increase in the order P4VP < P3VP < P2VP. This trend is likely due to varying electrostatic interactions along the respective polymeric chains, which hinder protonation of neighboring pyridine residues to differing extents; larger q values indicating greater repulsion. NMR studies conducted by Emsly [26] on pyridinium revealed that greater than 70% of positive charges are delocalized across the protons, a carbons, and their a hydrogens. Because these moieties are closer on P2VP than on P3VP, which in turn are closer than those on P4VP, repulsive interactions are greatest within P2VP and are least prominent within P4VP. In their studies of P2VP and P4VP, Satoh et al. [20] observed that P2VP solutions had higher viscosities than P4VP at a given a value, suggesting that P2VP adopts a more elongated conformation because of increased repulsion between charged sites. The q value of 1.0 obtained from titrations of P4VP with HClO4 indicate extensive counterion binding, thus significantly reducing repulsion between neighboring pyridinium residues; 
Differences in pKa,app at a given a, for a given polymer, are attributed to the varying extents to which the strong acid anions bind to protonated pyridine residues. Higher degrees of counterion condensation reduce the effective charge on the polycation, thus providing greater pKa,app values. These differences are exploited to assess the relative
Table 1. Values of q and pKa,app(0.55) for P2VP, P3VP, and P4VP titrations using HBr, HCl, HNO3, and HClO4 at 25˚C with no added salt.
extents to which Br−, Cl−, 





For all three polymers, Cl− is observed to the have the lowest shielding effect. As data in Table 2 indicate, Cl− has the smallest thermo chemical radius and largest free energy of dehydration among the anions investigated. It is also the most stabilizing anion of the four within the Hofmeister series [28] . In terms of a pseudo phase model, one could argue that its high dehydration energy imparts less partitioning of Cl− into the polyelectrolyte pseudo phase and, therefore, less effective shielding of the polymer’s cationic character.
Both Br− and 




The titration results for 



Table 2. Physical constants of poly(vinylpyridines) and selected anions [34] - [36] .
quaternized moieties [29] . In addition, in their studies of poly (allylammonium) with various counterions, Ochiai et al. [27] also observed that 


Several parameters have been suggested to correlate the extent to which various anions bind to cationic polyelectrolytes, some of which are provided in Table 2. Ther- mochemical radius and dehydration energies are commonly used in explaining binding phenomena. The results of Ochiai et al. [27] correlate well with the electron donor constant, En, an empirical parameter representing an anions hardness and polarizability. Burkhardt et al. [29] suggest using ionic polarizability values, but their results correlate poorly with


3.3. Polymer Titrations in the Presence of Added Salt
Table 2 also provides values for the intrinsic acid dissociation constants in the absence of neighboring electrostatic interactions, pK0, which were linearly extrapolated as the value of pKa,app at a = 0 in 0.10 M NaCl. Previous studies provided pK0 values for the monomer analogs 2-ethylpyridine and 4-ethylpyridine in 0.1 M NaCl, which are 6.04 and 6.14, respectively [20] . These values are considerably higher than those extrapolated for pyridine residues on the polymers studied. Kirsh et al. [33] attribute the decreases in pK0 values in polymers to lower effective dielectric constants associated with the local environment in the vicinity of the polymer backbone; the pyridine residue preferring to remain uncharged when in a medium of lower dielectric constant. However, no differences in pK0 were observed among the three polymers in 0.1 M NaCl despite the position of the nitrogen moving relative to the alkyl chain. This is likely a result of the mitigating influence of the added salt. Marked changes in ionic strength at low and high degrees of protonation prevent meaningful extrapolations of pK0 in the absence of added salt.
Figure 5 demonstrates the effect of added NaCl on the titration behavior of PVPs with HCl. The pKa,app values for all polymers increase across the a range investigated in the presence of NaCl, further illustrating that there are strong electrostatic repulsive forces between both neighboring pyridinium residues, and between those residues and
Figure 5. Titrations of P2VP, P3VP, and P4VP with HCl in the presence and absence of NaCl.
hydronium. The addition of competing Cl− counterions serves to reduce this repulsion, thus enhancing the ease with which the pyridine moieties are protonated and increasing pKa,app. Because of water’s high dielectric constant, these results are best explained in terms of electrostatic interactions taking place through the polymer as opposed to through the solution. Viscosity measurements of P2VP and P4VP solutions by Kirsh et al. [19] further illustrate this mechanism of interaction.
4. Conclusion
Potentiometric titrations have been used to investigate various aspects of the interactions of Br−, Cl−, 




Cite this paper
Roach, J.D., Bondaruk, M.M., Al-Abdulghani, A. and Shahrori, Z. (2016) Counterion Binding in Aq- ueous Solutions of Poly(vinylpyridines) as Assessed by Potentiometric Titration. Advances in Materials Physics and Chemistry, 6, 249-261. http://dx.doi.org/10.4236/ampc.2016.69025
References
- 1. Chen, J. and Chang, C. (2014) Fabrications and Applications of Stimulus-Responsive Polymer Films and Patterns on Surfaces: A Review. Materials, 7, 805-875.
http://dx.doi.org/10.3390/ma7020805 - 2. Cabane, E., Zhang, X., Langowska, K., Palivan, C. and Meier, W. (2012) Stimuli-Responsive Polymer and Their Applications in Nanomedicine. Biointerphases, 7, 9-36.
http://dx.doi.org/10.1007/s13758-011-0009-3 - 3. Stuart, M.A.C., Huck, W.T., Genzer, J., Muller, M., Ober, C., Stamm, M., Sukhorukow, G.B., Szleifer, I., Tsukruk, V.V., Urban, M., Winnik, F., Zauscher, S., Luzinov, I. and Minko, S. (2010) Emerging Applications of Stimuli-Responsive Polymer Materials. Nature Materials, 9, 101-113.
http://dx.doi.org/10.1038/nmat2614 - 4. Bajpai, A.K., Shukla, S.K., Bhanu, S. and Kankane, S. (2008) Responsive Polymers in Controlled Drug Delivery. Progress in Polymer Science, 33, 1088-1118.
http://dx.doi.org/10.1016/j.progpolymsci.2008.07.005 - 5. Schmaljohann, D. (2006) Thermo- and pH-Responsive Polymer in Drug Delivery. Advanced Drug Delivery Reviews, 58, 1655-1670.
http://dx.doi.org/10.1016/j.addr.2006.09.020 - 6. Sanjuan, S., Perrin, P., Pantoustier, N. and Tran, Y. (2007) Synthesis and Swelling Behavior of pH-Responsive Polybase Brushes. Langmuir, 23, 5769-5778.
http://dx.doi.org/10.1021/la063450z - 7. Atanase, L.I. and Riess, G. (2014) Stabilization of Non-Aqueous Emulsions by Poly (2-vinylpyridine)-b-Poly(butadiene) Block Copolymers. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 458, 19-24.
http://dx.doi.org/10.1016/j.colsurfa.2014.01.026 - 8. Atanase, L.I., Lerch, J.P. and Riess, G. (2015) Water Dispersibility of Non-Aqueous Emulsions Stabilized and Viscosified by Poly(butadiene)-Poly(2-vinylpyridine)-Poly(ethylene oxide) (PBut-P2VP-PEO) Triblock Copolymer. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 464, 89-95.
http://dx.doi.org/10.1016/j.colsurfa.2014.10.026 - 9. Motornov, M., Roiter, Y., Tokarev, I. and Minko, S. (2010) Stimuli-Responsive Nanoparticles, Nanogels and Capsules for Integrated Multifunctional Intelligent Systems. Progress in Polymer Science, 35, 174-211.
http://dx.doi.org/10.1016/j.progpolymsci.2009.10.004 - 10. Abdalla, S., Al-Marzouki, F., Obaid, A. and Gamal, S. (2016) Action of Colloidal Silica Films on Different Nano-Composites. Results in Physics, 6, 209-214.
http://dx.doi.org/10.1016/j.rinp.2016.04.014 - 11. Roach, J.D., Lane, R.L. and Hussain, Y. (2011) Comparative Study of the Uses of Poly (4-vinylpyridine) and Poly(diallyldimethylammonium) Chloride for the Removal of Perchlorate from Aqueous Solution by Polyelectrolyte-Enhanced Ultrafiltration. Water Research, 45, 1387-1393.
http://dx.doi.org/10.1016/j.watres.2010.10.027 - 12. Frank-Lacaze, L., Sistat, P., Huguet, P. and Lapicque, F. (2009) Protonation and Diffusion Phenomena in Poly(4-vinylpyridine)-Based Weak Anion-Exchange Membranes. Journal of Membrane Science, 340, 257-265.
http://dx.doi.org/10.1016/j.memsci.2009.05.046 - 13. Hong, S., Kim, M., Hong, C.K., Jung, D. and Shim, S.E. (2008) Encapsulation of Multi-Walled Carbon Nanotubes by Poly(4-vinylpyridine) and Its Dispersion in Various Solvent Media. Synthetic Metals, 158, 900-907.
http://dx.doi.org/10.1016/j.synthmet.2008.06.023 - 14. Mika, A.M. and Childs, R.F. (1999) Acid/Base Properties of Poly(4-vinylpyridine) Anchored within Microporous Membranes. Journal of Membrane Science, 152, 129-140.
http://dx.doi.org/10.1016/S0376-7388(98)00219-1 - 15. Jewett, D.M. and Ehrenkaufer, R.L. (1982) Rapid and Efficient Preparation of Free Amino Acids from Strong Acid Salts on Columns of Crosslinked Poly(4-vinylpyridine). Analytical Biochemistry, 122, 319-321.
http://dx.doi.org/10.1016/0003-2697(82)90289-5 - 16. Oosawa, F. (1957) A Simple Theory of Thermodynamic Properties of Polyelectrolyte Solutions. Journal of Polymer Science Part A: Polymer Chemistry, 23, 421-430.
http://dx.doi.org/10.1002/pol.1957.1202310335 - 17. Manning, G.S. (1969) Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions. The Journal of Chemical Physics, 51, 924-933.
http://dx.doi.org/10.1063/1.1672157 - 18. Fuoss, R.M. and Strauss, U.P. (1948) Polyelectrolytes. II. Poly-4-vinylpyridonium Chloride and Poly-4-vinyl-N-n-butylpyridonium Bromide. Journal of Polymer Science Part A: Polymer Chemistry, 3, 246-263.
http://dx.doi.org/10.1002/pol.1948.120030211 - 19. Kirsh, Y.E., Komarova, O.P. and Lukovkin, G.M. (1973) Physico-Chemical Study of Ionic Equilibria for Poly-2 and Poly-4-vinylpyridines in Alcohol-Water Solutions. European Polymer Journal, 9, 1405-1415.
http://dx.doi.org/10.1016/0014-3057(73)90110-9 - 20. Satoh, M., Yoda, E., Hayashi, T. and Komiyama, J. (1989) Potentiometric Titration of Poly(vinylpyridines) and Hydrophobic Interaction in the Counterion Binding. Macromolecules, 22, 1808-1812.
http://dx.doi.org/10.1021/ma00194a051 - 21. Yoshida, M. (1997) Solution Properties of Polyvinylpyridine in Acid-II. Solution Properties of Poly(4-vinylpyridine) in Aqueous Solution of Hydrochloric Acid. European Polymer Journal, 33, 943-948.
http://dx.doi.org/10.1016/S0014-3057(96)00140-1 - 22. Roach, J.D. and Tush, D. (2008) Equilibrium Dialysis and Ultrafiltration Investigations of Perchlorate Removal from Aqueous Solution Using Poly(diallyldimethylammonium) Chloride. Water Research, 42, 1204-1210.
http://dx.doi.org/10.1016/j.watres.2007.09.003 - 23. Ge, Q., Su, J., Amy, G.L. and Chung, T. (2012) Exploration of Polyelectrolytes as Draw Solutes in Forward Osmosis Processes. Water Research, 46, 1318-1326.
http://dx.doi.org/10.1016/j.watres.2011.12.043 - 24. Gans, P. and O’Sullivan, B. (2000) GLEE, a New Computer Program for Glass Electrode Calibration. Talanta, 51, 33-37.
http://dx.doi.org/10.1016/S0039-9140(99)00245-3 - 25. Lewis, E.A., Barkley, T.J., Reams, R.R. and Hansen, L.D. (1984) Thermodynamics of Proton Ionization from Poly(vinylammonium Salts). Macromolecules, 17, 2874-2881.
http://dx.doi.org/10.1021/ma00142a073 - 26. Emsley, J.W. (1968) A Semi-Empirical Self Consistent Field Molecular Orbital Calculation on Pyridine and Pyridinium Ion Including All Valence Electrons. Journal of Chemical Society A: Inorganic, Physical, Theoretical, 1387-1393.
http://dx.doi.org/10.1039/j19680001387 - 27. Ochiai, H., Anabuki, Y., Kojima, O., Tominaga, K. and Murakami, I. (1990) Dissociation of Poly(allylammonium) Cations in Salt Solutions. Journal of Polymer Science: Part B: Polymer Physics, 28, 233-240.
http://dx.doi.org/10.1002/polb.1990.090280209 - 28. Salis, A. and Ninham, B.W. (2014) Models and Mechanisms of Hofmeister Effects in Electrolyte Solutions, and Colloid and Protein Systems Revisited. Chemical Society Reviews, 43, 7358-7377.
http://dx.doi.org/10.1039/C4CS00144C - 29. Burkhardt, C.W., Parazak, D.P., McCarthy, K.J. and Jackson, G.J. (1986) Specific Counterion Binding to Cationic Polyelectrolytes. Journal of Applied Polymer Science, 32, 4701-4708. http://dx.doi.org/10.1002/app.1986.070320434
- 30. Tirumalesh, K. (2008) Simultaneous Determination of Bromide and Nitrate in Contaminated Waters by Ion Chromatography Using Amperometry and Absorbance Detectors. Talanta, 74, 1428-1434.
http://dx.doi.org/10.1016/j.talanta.2007.09.021 - 31. Huq, H.P., Yang, J.S. and Yang, J.W. (2007) Removal of Perchlorate from Groundwater by the Polyelectrolyte-Enhanced Ultrafiltration Process. Desalination, 204, 335-343.
http://dx.doi.org/10.1016/j.desal.2006.02.039 - 32. Cook, J.P. and Riley, D.J. (2009) The Effect of Perchlorate Ions on a Pyridine-Based Microgel. Advances in Colloid and Interface Science, 147, 67-73.
http://dx.doi.org/10.1016/j.cis.2008.11.002 - 33. Kirsh, Y.E. (1985) Reactivity and Physico-Chemical Properties of Nitrogen-Containing Carbon-Chain Polymers in Aqueous Solutions. Progress in Polymer Science, 11, 283-338.
http://dx.doi.org/10.1016/0079-6700(85)90010-3 - 34. Moyer, B. and Bonnesen, P.V. (1979) Physical Factors in Anion Separation. In: Bianchi, A., Bowman-James, K. and Garcia-Espana, E., Eds., Supramolecular Chemistry of Anions, Wiley-VCH, Inc., New York, 1-44.
- 35. Yamada, S. and Tanaka, M. (1975) Softness of Some Metal Ions. Journal of Inorganic and Nuclear Chemistry, 37, 587-589.
http://dx.doi.org/10.1016/0022-1902(75)80387-3 - 36. Edwards, J.O. (1956) Polarizability, Basicity and Nuleophilic Character. Journal of the American Chemical Society, 78, 1819-1820.
http://dx.doi.org/10.1021/ja01590a012







