Open Journal of Ecology
Vol.06 No.02(2016), Article ID:62832,7 pages
10.4236/oje.2016.62006
Examination of the Level of Heavy Metals in Wastewater of Bandar Abbas Wastewater Treatment Plant
Gholamreza Mansourri*, Mehrzad Madani
Science in Environmental Engineering, Islamic Azad University, Tehran, Iran

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 December 2015; accepted 16 January 2016; published 19 January 2016
ABSTRACT
One of the main environmental pollutants is heavy metals. Due to extensive usage in industry, these metals enter biological cycle rapidly and contaminated water and soil resources rapidly. In this work, lead, copper, zinc and chromium of Bandar Abbas wastewater are examined. For this research, nine stations were set for measurement in urban level in Bandar Abbas and sampling of aforesaid metals was performed in fall and winter 2006 in these stations. After extraction and preparation operations using APDC-MIBK, samples were measured using flame atomic absorption system. According to results, concentrate of studied metals was lower than allowable standard value set by Iran environmental protection organization for agricultural purposes and sewage to ground level waters. In addition, efficiency of Bandar Abbas wastewater treatment plant to remove these metals is 40% - 70% from which highest removal is for zinc as much as 71.1% and lowest level is for copper as much as 40.5%. However, copper level was higher than allowable level for agricultural purposes in spring and summer (0.21 mg/L and 0.23 mg/L, respectively) and lower in fall and winter (0.103 mg/L and 0.098 mg/L, respectively). Furthermore, changes in concentration of metals in these stations in various seasons were measured and analyzed using one- way variance analysis and simultaneous effects of time and place on measured variables were analyzed using two-way variance analysis.
Keywords:
Urban Wastewater, Bandar Abbas, Heavy Metals, Treatment Plant

1. Introduction
Necessity of sustainable development is to take care of planet and protect environment either as appropriate care or as sound usage of natural resources. One of the problems which is caused by urban and industrial development is the issue of production of various wastes in the form of solid, semi-solid, liquid or gas and many countries face problems in their management, design, production, utilization and maintenance of systems of collection, treatment and sewage of these wastes and require comprehensive and applied solutions [1] . In such circumstances, reuse of wastewater and finding new alternative resources for water for various usages is one of the ways of solving water shortage problem and hence, reuse of wastewater for agricultural, industrial, recreational and feed of underground waters and growing aquatics must be one of the main goals of wastewater treatment projects [2] . On the other hand, using wastes of wastewater treatment is one of the valuable resources owing to presence of compounds which strengthens soil and water ecosystems and must not be ignored [1] .
One of the pollutants is heavy metals. Due to toxicity and cumulative effects in the body of organisms, resistance to decomposition and biological reactions, these pollutants impose destructive effects on environment and by entering food chain, put organisms and ultimately human into the threat of toxicity, cancer and long-term and short-term genetic effects [3] . At the end, the main purpose of this research is explained as follows:
1) Measurement of heavy metals including copper, lead, zinc, nickel and chromium in Bandar Abbas city wastewater.
2) Determination of the quality of outlet wastes of Bandar Abbas wastewater treatment plant with respect to above heavy metals and comparing them with standards of Iran environmental protection organization.
3) Studying the average changes in concentration of heavy metals in various seasons of the year.
4) Examination of the average changes in concentration of heavy metals in various stations (before treatment, inlet and outlet of treatment and next stations).
5) Evaluation of the efficiency of wastewater treatment plant for removal of these metals and assessing the feasibility of reuse of refined wastes.
6) Investigation and study of simultaneous effects of time and place on measured variables.
2. Literature Review
Mapanda et al. showed in a research in Zimbabwe that using wastewater for irritation of gardens and vegetables leads to enrichment of soil from heavy metals and can threaten environment and health in long-term [4] . Moreover, Constantino et.al estimated the level of accumulation of rare elements such as boron, cadmium, chromium, mercury, lead and arsenic in agricultural soil of Hidalgo State in Mexico and their results revealed that cadmium and lead level is higher than allowable limit determined in German and Netherlands standards. It means that soil of this region is in danger [5] . Finally, JalilZadeg and Parvaresh studied the level of heavy metals in wastes of southern Isfahan wastewater treatment plant wastes and their obtained data suggested that highest level was for lead, nickel, copper and chromium as much as 0.609, 0.610, 0.484, 0.990 mg/L and three months average for these metals was as much as 0.345, 0.242, 0.331 and 0.475 mg/L, respectively [6] .
3. Heavy Metals
Heavy metals term refers to a group of elements which have a specific weight higher than 6 gr/cm3 and atomic mass more than 50. Further, each caution with atomic weight higher than 23 (corresponding to sodium) are called heavy metal. In general, heavy metals include metal elements whose atomic number is higher than 40. In this way, soil base metals, base metals, lanthanides and actinides are excluded from this definition. Metals with specific weight more than 5 gr/cm3 are called heavy metals. These metals are classified into necessary and unnecessary groups. Some of them are considered as necessary elements in minor portions and are required for biological growth of organisms and their deficiency can be limiting; such as copper for growth of algae. In fact, presence of minor portions of them in food of human and other organisms is necessary. However, same metals in higher than allowable limit concentrations will make various unsuitable consequences for human as well as other organisms while they result in environmental pollutions and threats as well [3] .
Unnecessary metals are those which are dangerous for human and other organisms even in lower concentrations. Of necessary metals, cobalt, copper, iron, selenium, manganese, molybdenum, zinc can be noted and some unnecessary metals are mercury, cadmium, lead and silver which are dangerous even in low concentrations. Hence, some of heavy metals are necessary, some are toxic and depending on concentration, some of them are toxic or useful.
4. Toxicity of Heavy Metals
Minor values of these metals constitute significant portions of wastewaters. Most of these metals are classified as first degree pollutants. US environmental protection organization recognized about 129 first degree pollutants in 65 groups which must follow definite standards of wastewater sewage [7] . Uncontrolled and serious increase in production and consumption of these metals in recent decades led to their entrance into aquatic environments and as a result of resistance to biological decomposition, they made destructive effects the most important of which can be noted as consequences and problems such as carcinogen, affecting central and peripheral nervous system, effect on skin, hematopoietic system, damage to kidneys and accumulation in body tissues and so on. In general, toxic effects of metals correspond to nonspecific reactions with sulfhydryl, carboxyl, phosphate, amine and other groups or entrance of metals to cellular macromolecules [2] . Toxicity of various metals for human is as follows:
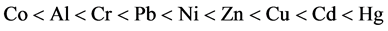
5. Materials and Methods
In this research, there are 9 sampling stations. Samplings are performed in four times during spring, summer, fall and winter 2006-2007. In each time, three samples were taken from each station and totally, 108 samples were collected. Moreover, sampling and storage of samples are performed according to procedure 3010B of the book “standard methods of test of water and wastewater”. In this research, 1 L polyethylene containers are used for sampling [8] . To prevent volume change due to evaporation, samples are kept in refrigerator in 4˚C and in such conditions; samples can be kept for 6 months [8] . To remove solid particles from wastewater, samples were passes from filter. To perform preparation and extraction, procedure 3111C of standard methods is used. This method is appropriate for low concentrations of metals. This method leads to concentration of the sample and hence, detection-limit increase. To perform extraction, 500 mL of the sample is poured in separating funnel. 5 mL of APDC solution is added to it (APDC must be 1% of the sample). 20 mL of MIBK solution is added to it and is mixed strongly for 30 s. It must be noted that maximum volume of sample to MIBK is 40. Compound is allowed to be separated to water and organic level. Bottom layer is discarded and organic layer is picked. If during extraction operation, an emulsion is formed in MIBK-water interface, dehydrated Na2SO4 is added to form a homogeneous organic phase. Of course, we did not observe such issue in extraction operation and obtained organic layer was homogeneous [8] . After this stage, since measurement was not possible immediately after extraction, obtained organic phase was transferred again to aquatic phase so that it can be possible to keep it.
Process stages are as follows: first, we allow organic phase to evaporate in ambient temperature. Then, evaporation products are solved in 0.5 mL thick nitric acid and transferred to 25 mL volumetric flask and reaches volume using distilled water. Obtained solution can be kept in refrigerator. Method used for measuring intended metals is flame absorption spectrometry and it was performed by VARIAN SpectAA 220 model. Stages of experiment are in accordance with procedure 3110 of standard as well as instructions of the system [8] [9] . To calibrate system, standard solutions (tetrazolium) produced by Merck company was used. To statistically analyze data, Stat Graphics Plus 3.0 and to sketch diagrams, Microsoft Excel 2003 was used. To statistically analyze data, changes of investigated parameters in various stations and seasons were evaluated using one-way variance analysis and to investigate and study of the simultaneous effect of time and place on measured variables, two-way variance analysis was used. In cases in which difference is significant, to find the source of difference, Duncan multiple comparison method is used. Finally, to compare obtained results, standards of Iran environmental protection organization corresponding to wastewater sewage and reuse of wastes is used [10] .
6. Results
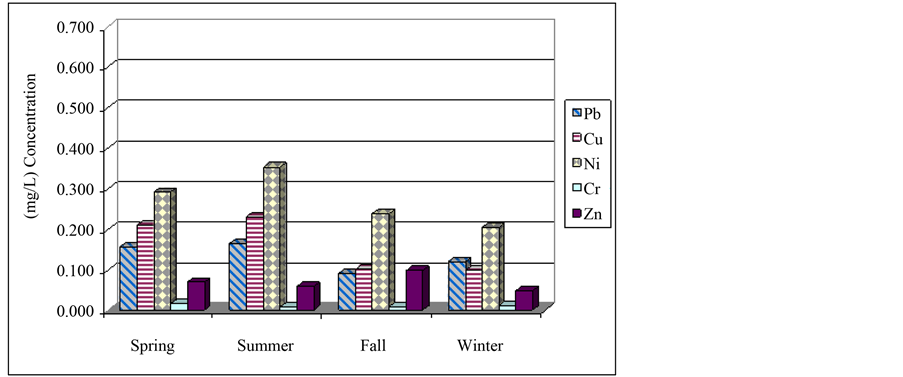
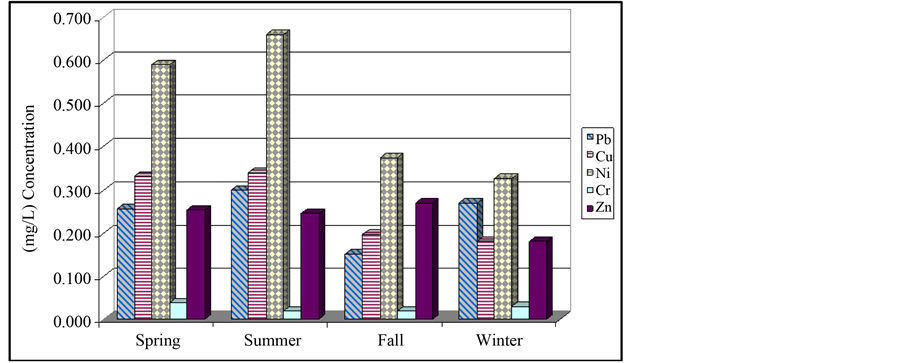
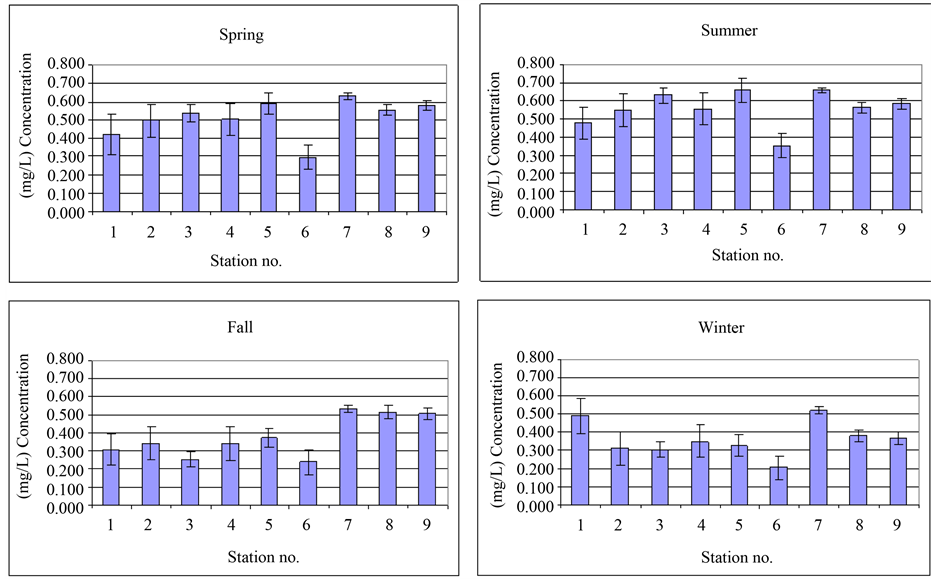
Figure 1 and Figure 2 represent the average measured concentration of metals in various stations and Figure 3 illustrates the concentrations in various seasons. In Figure 4, comparison of average concentration of heavy metals in inlet wastewater an outlet wastes of Bandar Abbas wastewater treatment plant with standards of Iran
Figure 1. Average concentration of heavy metals in station 6 of Bandar Abbas treatment plant.
Figure 2. Average concentration of heavy metals in station 5 of Bandar Abbas treatment plant.
environmental protection organization. Summary of results of one-way variance to investigate the changes in concentration of metals in various seasons and stations are presented in Table 1 and Table 2. Results of investigation of simultaneous effect of time and place on measured concentration of metals using two-way variance analysis are shown in Table 3. Table 3 represents the efficiency of Bandar Abbas wastewater treatment plant in removing heavy metals and Table 4 shows average concentration of heavy metals in outlet wastes of treatment plant in various seasons, their annual average and its comparison with standard values for Iran for different usages.
7. Results and Discussion
By one-way variance analysis, changes of average of concentration of each of the metals in various seasons are investigated. According to results, P-value is calculated in 95% confidence level for chromium, zinc and lead as much as 0.2394, 0.4303 and 0.0781, respectively and since calculated values are higher than 0.05, there is a significant difference between average of results in various seasons. Results of Duncan test for source of difference suggest that there is significant difference between four pairs of result in 95% confidence level. These four pairs are: spring-fall, summer-fall, spring-winter and summer-winter. Moreover, this test represents two homogeneous groups: spring-summer and fall-winter. Obtained results for nickel are completely similar to copper. Table 1
Figure 3. Average concentration of nickel in various seasons.
Figure 4. Comparison of average concentration of heavy metals in outlet wastewater and outlet wastes of wastewater treatment plant with standard values of environmental protection organization.
Table 1. e-value and F-ratio for metals in various seasons.
Table 2. e-value and F-ratio for metals in various stations.
Table 3. Summary of results of one-way ANOVA.
Table 4. Measured concentration of metals in inlet wastewater and outlet wastes of treatment plant and percent of removal.
shows P-value and F-ratio for average of concentration of metals using one-way variance analysis with 95% confidence level in various seasons of the year. Then, using two-way variance analysis, simultaneous effects of time and place on metals is studied. Summary of analysis performed in this stage is presented in Table 3. Since p-value of each of the cases is less than 0.05, average of concentration of metals has significant difference in 95% confidence level. In other words, both factors of time and place have statistically significant on concentration of measured parameters. To find the origin of difference, Duncan method is used and yielded following results: for nickel, in four pairs of time, there is a significant difference. These pairs are as follows: spring-fall, spring-win- ter, summer-fall, summer-winter. Two homogeneous groups are: spring-summer, fall-winter. Moreover, among all 12 pairs, difference is significant. These pairs are: station 6 with stations 1, 2, 3, 4, 5, 7, 8, 9, station 7 and stations 1, 2, 3, 4. There are three homogeneous groups with respect to place: group one (station 6), groups 2 (stations 1, 2, 3, 4, 5, 8 and 9) and group three (station 5, 7, 8, 9). Moreover, in Table 4, average of concentration of heavy metals in inlet wastewater and outlet wastes of Bandar Abbas wastewater treatment plant in various seasons together with annual average and standard deviation of them as well as the efficiency of plant in removing these metals are represented. According to results of this table, removal level of metals lead, copper, nickel, chromium and zinc is 44.7%, 40.5%, 42.5%, 55.1% and 71.1%, respectively. As can be observed, least efficiency corresponds to removal of copper (40.5%) and highest level corresponds to zinc (71.1%). As stated earlier, common systems of treatment of wastewater don’t show appropriate efficiency for removal of heavy metals and this fact is confirmed by various researches including present paper [6] [11] . Finally, concentration of studied heavy metals in outlet wastes of treatment plant in various seasons, their annual average as well as standard of Iran environmental protection organization corresponding to sewage of wastewater and reuse of waste are compared in Table 5. According to results of table, average concentration of metals lead, zinc, chromium and nickel is considerably lower than allowable limit for various usages and reuse of wastes is not problematic. However, with respect to copper, it is observed that in spring and summer, measured values are slightly higher than allowable limit for agricultural and irritation usages. Of course, obtained concentrations show significant reductions in fall and winter and are in agreement with corresponding standard. Anyway, according to instructions, copper content in the range of 0.1 - 1 mg/L can be harmful and toxic for many plants [1] . Therefore, when irritating with this waste, selection of the type of plant must be done with caution. Figure 4 illustrates the comparison of concentration of heavy metals in inlet wastewater and outlet wastes of wastewater treatment plant with standard values of Iran environmental protection organization.
Table 5. Comparison of concentration of heavy metals according to standard of Iran environmental protection organization.
*Chromium III. **Chromium IV.
*Corresponding author.
According to discussions and presented diagrams, it can be concluded that it is possible to reuse wastes of wastewater treatment plant for sewage to groundwater, absorption well as well as using in irritation and agriculture with respect to investigated parameters and as stated earlier, it is only necessary to take care of selection of plant type for the case ofcopper. With respect to efficiency for the plant for removal of heavy metals, this treatment plant has appropriate performance compared to similar systems of treatment. According to Table 2 and significant difference observed in one-way variance analysis of measurement stations for lead, zinc and chromium and as explained previously that difference corresponds to pairs of before and after treatment plant, it can be inferred that treatment has a relatively suitable efficiency in removal of metals. Referring to Table 4, it is clear that highest level of removal pertains to these metals.
Cite this paper
Gholamreza Mansourri,Mehrzad Madani, (2016) Examination of the Level of Heavy Metals in Wastewater of Bandar Abbas Wastewater Treatment Plant. Open Journal of Ecology,06,55-61. doi: 10.4236/oje.2016.62006
References
- 1. Abedi, M. and Najafi, P. (2001) Using Treated Wastewater in Agriculture. National Committee of Irritation and Drainage of Iran, Vol. 47.
- 2. Ghaneian, M.T., Mesdaghi Nia, A.R. and Ehrampoush, M.H. (2001) Principles of Reuse of Wastewater, Principles, Methods. Standards and Health Threats, University of Medical Sciences, Teb Gostar Press.
- 3. Eidan, G. (2002) Biologic Treatment of Urban—Industrial Wastes, Human Resources Training of Iran National Gas Company.
- 4. Mapanda, F., Mangwayana, E.N., Nyamangara, J. and Giller, K.E. (2005) The Effect of Long-Term Irrigation Using Wastewater on Heavy Metal Content of Soil under Vegetable in Harara, Zimbabwe. Agriculture, Ecosystem & Environment, 107, 151-165.
http://dx.doi.org/10.1016/j.agee.2004.11.005 - 5. Constantino, C.A.L., Garsia, F.P. and Del Razo, L.M. (2005) Chemical Fractionation of Boron and Heavy Metals in Soils Irrigated with Wastewater in Central Mexico. Agriculture, Ecosystem & Environment, 108, 57-71.
http://dx.doi.org/10.1016/j.agee.2004.12.013 - 6. Jalil Zadeh, A. and Parvaresh, A. (2006) Heavy Metals in Wastes of Southern Isfahan Wastewater Treatment Plant. Water and Environment, 65, 35-39.
- 7. Metcalf & Eddy, Inc. (1991) Wastewater Engineering, Treatment, Disposal, Reuse. 3rd Edition, McGraw Hill.
- 8. APHA-AWWA-WEF (2005) Standard Method for the Examination of Water and Wastewater. 21st Edition, American Public Health Association, American Water Works Association, Water Environmental Federation, Washington DC.
- 9. Varian Flame Atomic Spectrometry (Revised) (1989) Analytical Methods. SpectAA 220.
- 10. Iran Environmental Protection Organization (1999) Environmental Regulations and Standards. Environment Organization Press.
- 11. Chipasa, K.B. (2003) Accumulation and Fate of Selected Heavy Metals in a Biological Wastewater Treatment System. Waste Management, 23, 135-143.
http://dx.doi.org/10.1016/S0956-053X(02)00065-X
NOTES
*Corresponding author.