Open Journal of Genetics
Vol.1 No.2(2011), Article ID:7308,4 pages DOI:10.4236/ojgen.2011.12002
PAH mutational spectrum: still expanding
![]()
1Medical Genetics Center, National Institute of Health, INSA, Porto, Portugal;
2IPATIMUP, Institute of Molecular Pathology and Immunology of the University of Porto, Porto, Portugal;
3Faculty of Medicine of the University of Porto, Porto, Portugal;
4Faculty of Sciences of the University of Porto, Porto, Portugal.
Email: *laura.vilarinho@insa.min-saude.pt; *lazevedo@ipatimup.pt
Received 4 May 2011; revised 30 June2011; accepted 15 July 2011.
Keywords: Phenylketonuria (PKU); Phenylalanine Hydroxylase; PAH Gene; Novel Mutation
ABSTRACT
Phenylketonuria (PKU, MIM 261600) is the most common inborn error of amino acid metabolism. To date, a total of more than 500 mutations have been associated with the disease. In this report, the novel p.Glu182Lys mutation, found in a Portuguese family in combination with the previously reported p.Leu 348Val, is presented and its putative deleterious impact discussed.
1. INTRODUCTION
Phenylketonuria is the most common inborn error of amino acid metabolism, occurring in approximately 1 in 10,000 births among Caucasians [1]. It is caused by the impairment of the hepatic enzyme phenylalanine hydroxylase (PAH, EC 1.14.16.1) and inherited as a Mendelian autosomal recessive disease. Failure to convert phenylalanine to tyrosine leads to an increase of phenylalanine in body fluids which is responsible for growth failure, seizures, microcephaly, mental retardation and intellectual impairment [2]. As result of the neonatal screening programs, affected patients are identified early and phenylalanine-poor diets implemented to prevent the appearance of PKU-related symptoms.
At the genetic level, PKU is caused by mutations in PAH gene located on q22 - q24.1 region of chromosome 12 [3]. To date, a total of more than 500 mutations are listed in public databases [4,5]. The distribution and relative frequencies of the PAH gene mutations have been reported in various populations and current data reveal a large degree of heterogeneity in the PAH mutational spectrum [6-13]. Still, there are some mutations that occur more frequently associated with specific populations as is the case of p.Arg270Lys, p.Val388Met, p.Phe410Cys and IVS11 + 5 G > A, whose origin is frequently associated with the Iberian population. The most recent case refers to the p.Phe410Cys which was firstly detected in a Portuguese family [14] and thereafter also found in a Brazilian patient [15]. Because no haplotypic data were available, conclusive data on a common origin is not possible, although this hypothesis seems very likely taking into account the Brazilian population history.
Herein, we report a novel mutation detected in the PAH gene in a family of Portuguese ancestry. To evaluate the genotype-phenotype association we have combined data on the nature of amino-acid replacement and the conservation degree of the residue in non-human PAH homologues, which allowed us to predict the deleterious effect of the novel p.Glu182Lys replacement.
2. MATERIAL AND METHODS
2.1. Patient Screening
Until the end of 2010, 3.072.774 newborns were screened in National Neonatal Screening Program. Among those 280 were assigned as PKU patients. After obtained informed consent, 160 PKU patients were further studied at the molecular level. All the mutations found were previously identified and recorded in the literature, with the exception of the replacement here discussed.
The patient reported here is a female that was born in 1990 from non-consanguineous parents. At day seven of life, a blood sample was collected and the phenylalanine level was determined (600 µmol/L). The patient started a phenylalanine-poor diet protocol in order to avoid PKU symptoms.
2.2. Molecular Analyses
Genomic DNA was automatically extracted from dried blood spots (EZ1 DNA Tissue kit, QIAGEN). Polymerase chain reaction (PCR) amplification of the PAH exons using flanking intronic primers were performed followed by automatic sequencing in an ABI PRISM 3130XL Genetic Analyser (Applied Biosystems). Primer sequences are detailed in Table 1.
PCR was performed as follows: initial denaturation (95˚C, 10 min); 30 cycles of denaturation (95˚C, 1 min), annealing (60˚C, 1 min) and extension (72˚C, 1 min), and a final extension at 72˚C for 5 min. All primers were designed with M13 F/R tail labeled. The sequence of M13F tail is 5’TGTAAAACGACGGCCAGT3’ and the M13R tail is 5’CAGGAAACAGCTATGACC3’.
Amplified products were purified with ExoSAP-IT (USB Corporation, Ohio, USA) by incubation at 37˚C for 15 min and followed by enzyme inactivation for 15 min at 85˚C. The sequencing reaction was performed with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) using the M13F and the M13R primers. Sequencing reaction conditions were the following: initial denaturation at 94˚C for 2 min followed by 25 cycles of denaturation (94˚C, 10 sec), annealing (50˚C, 10 sec) and extension (60˚C, 4 min). The fragments resulting from sequencing reaction were purified with DyeEx 96 kit (QIAGEN) and sequenced in an ABI3130 XL automated sequencer.
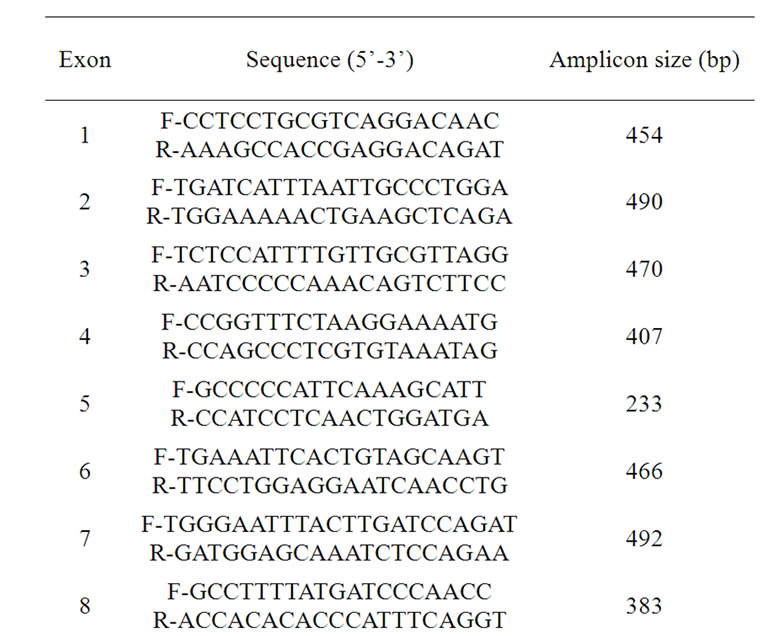

Table 1. Primers sequences and PCR conditions for the amplification of PAH gene.
2.3. Interspecies Comparative Analysis
Sequences from vertebrate PAH homologs were obtained in the Ensembl database [16] and aligned in Muscle [17] running in the Geneious v5.0.4 software [18].
3. RESULTS AND DISCUSSION
Here we present the molecular diagnostic screening of a female PKU patient who revealed an increased value of phenylalanine level at birth during the routine newborn screening. The analysis was based on a PCR-based strategy that resulted in the amplification of all exons of the PAH gene. A novel p.Glu182Lys replacement was found in heterozygozity with p.Leu348Val and both are located in the catalytic domain of the protein. Although this mutation is not yet documented, a different replacement (p.Glu182Gly) was previously found in the same residue [19] reinforcing the hypothesis of a putative deleterious impact when the wild-type Glu182 is replaced by a differently charged residue. Although the change involves the same residue, in p.Glu182Lys the first nucleotide of 182 codon is affected (c.544 G > A), while in previously described p.Glu182Gly is the second position (c.545 A > G). When the novel p.Glu182Lys was analyzed in Polyphen and SIFT [20,21], a deleterious effect was predicted in both programs. In agreement, the conservation degree analysis across vertebrates (Figure 1) shows that although the residue 182 is not fully

Figure 1. Comparative analysis record showing PAH residues 182 and 348 in vertebrate species.
conserved across the entire vertebrate group (in fishes and amphibians Asp and Gln are wild-type alleles) the site is never occupied by a positive charged residue in either homolog. The second mutation (p.Leu348Val) lies in an invariant residue across the entire lineage. Altogether, molecular findings in combination with the biochemical PKU associated phenotype strongly support a deleterious effect of the novel p.Glu182Lys replacement.
4. CONCLUSIONS
In the era of genome-wide studies, reporting novel mutations in highly variable genes remains of extreme importance. Such information is expected to provide a better understanding of the mutational spectrum of the corresponding gene and also be useful when recurrent occurrence of the mutation allows phenotype comparisons among affected individuals. However, mutation identification alone is often not enough to allow predictions on the severity of observed phenotypes, that is, even for simple Mendelian disorders, such as PKU, genotypephenotype correlations may be complex to infer. In fact, phenotypes are not only the result of the causal mutations but also environment factors and epistatic interactions between distinct alleles [22,23]. In this sense, other variants linked in cis to the mutation site may influence and even explain frequently observed inconsistencies in the genotype-phenotype relationship [24]. Phenylketonuria can be considered such a case. Thus far, more than 500 mutations are documented in the PAH gene. For over two years no novel mutations have been reported which may indicate that the large mutational spectrum of the PAH gene is reaching a limit. The novel mutation herein reported is contributing to enlarge the group of detectable mutations in the PAH gene.
5. ACKNOWLEDGEMENTS
This work was supported by the Fundação para a Ciencia e a Tecnologia (FCT) research project SFRH/BD/44264/2008 and by POPHQREN-Tipologia 4.2- Scientific employment promotion funded by the European Social Fund and by national funds from the Ministry of Science, Technology and Higher Education (MCTES) to L.A. The Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP) is an Associate Laboratory of the Portuguese MCTES and is partially supported by FCT.
REFERENCES
- Loeber, J.G. (2007) Neonatal screening in Europe; the situation in 2004. Journal of Inherited Metabolic Disease, 30, 430-438. doi:10.1007/s10545-007-0644-5
- Williams, R.A., Mamotte, C.D. and Burnett, J.R. (2008) Penhylketonuria: An inborn error of phenylalanine metabolism. Clinical Biochemistry, 29, 31-41.
- Woo, S.L., Lidsky, A.S., Gütler, F., Chandra, T. and Robson, K.J. (1983) Cloned human phenylalanine hydroxylase gene allows prenatal diagnosis and carrier detection of classical phenylketonuria. Nature, 306, 151-155. doi:10.1038/306151a0
- Scriver, C.R., Hurtubise, M., Konecki, D., Phommarinh, M., Prevost, L., Erlandsen, H., Stevens, R., Waters, P.J., Ryan, S., McDonald, D. and Sarkissian, C. (2003) PAHdb 2003: What a locus-specific knowledgebase can do. Human Mutation, 21, 333-334.http://www.pahdb.mcgill. Ca
- Human Genetic Mutation Database.
http://www.hgmd.cf.ac.uk/ - Lee, D.H., Koo, S.K., Lee, K.S., Yeon, Y.J., Oh, H.J., Kim, S.W., Lee, S.J., Kim, S.S., Lee, J.E., Jo, I. and Jung, S.C. (2004) The molecular basis of phenylketonuria in Koreans. Journal of Human Genetics, 49, 617-621. doi:10.1007/s10038-004-0197-5
- Daniele, A., Cardillo, G., Pennino, C., Carbone, M.T., Scognamiglio, D., Correra, A., Pignero, A., Castaldo, G. and Salvatore, F. (2007) Molecular epidemiology of phenylalanine hydroxylase deficiency in Southern Italy: A 96% detection rate with ten novel mutations. Annals of Human Genetics, 71, 185-193. doi:10.1111/j.1469-1809.2006.00328.x
- Zschocke, J. (2003) Phenylketonuria mutations in Europe. Human Mutation, 21, 345-356. doi:10.1002/humu.10192
- Kasnauskiene, J., Giannattasio, S., Lattanzio, P., Cimbalistiene, L. and Kucinskas, V. (2003) The molecular basis of phenylketonuria in Lithuania. Human Mutation, 21, 398-403. doi:10.1002/humu.9113
- Zschocke, J. and Hoffmann, G.F. (1999) Phenylketonuria mutations in Germany. Human Mutation, 104, 390-398. doi:10.1007/s004390050973
- Guldberg, P., Levy, H.L., Hanley, W.B., Koch, R., Matalon, R., Rouse, B.M., Trefz, F., De la Cruz, F., Henriksen, K.F. and Guttler, F. (1996) Phenylalanine hydroxylase gene mutations in the United States: Report from the Maternal PKU Collaborative Study. The American Journal of Human Genetics, 59, 684-694.
- Eiken, H.G., Knappskog, P.M., Boman, H., Thune, K.S., Kaada, G., Motzfeldt, K. and Apold, J. (1996) Relative frequency, heterogeneity and geographic clustering of PKU mutations in Norway. European Journal of Human Genetics, 4, 205-213.
- Rivera, I., Leandro, P., Lichter-Konecki, U., Almeida, I.T. and Lechner, M.C. (1998) Population genetics of hyperphenylalaninaemia resulting from phenylalanine hydroxylase deficiency in Portugal. Journal of Medical Genetics, 35, 301-304. doi:10.1136/jmg.35.4.301
- Vilarinho, L., Queirós, A., Leandro, P., Almeida, I.T. and Rivera I. (2006) Fenilcetonúria revisitada. Arquivos de Medicina, 20, 161-171.
- Santos, L.L., Castro-Magalhães, M., Fonseca, C.G., Starling, A.L.P., Januário, J.N., Aguiar, M.J.B. and Carvalho, M.R.S. (2008) PKU in minas Gerais state, Brazil: Mutation analysis. Annals of Human Genetics, 72, 774-779. doi:10.1111/j.1469-1809.2008.00476.x
- Ensembl Genome Browser. http://www.ensembl.org
- Edgar, R.C. (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792-1797. doi:10.1093/nar/gkh340
- Drummond, A.J., Ashton, B., Buxton, S., Cheung, M., Cooper, A., Heled, J., Kearse, M., Moir, R., Stones-Havas, S., Sturrock, S., Thierer, T. and Wilson A. (2010) Inspirational Software for Biologiests, Geneious. http://www.geneious.com
- Eisensmith, R.C. and Woo, S.L. (1995) Molecular genetics of phenylketonuria: From molecular anthropology to gene therapy. Advances in Genetics, 32, 199-271. doi:10.1016/S0065-2660(08)60206-0
- Polyphen: Prediction of functional effect of human nsSNPs. http://genetics.bwh.harvard.edu/pph
- Sorting Intolerant from Tolerant (SIFT). http://blocks.fhcrc.org/sift/SIFT.html
- Dipple, K.M. and McCabe, E.R.B. (2000) Phenotypes of patients with “simple” mendelian disorders are complex traits: Thresholds, modifiers, and systems dynamics. The American Journal of Human Genetics, 66, 1729-1735. doi:10.1086/302938
- Scriver C.R. and Waters, P.J. (1999) Monogenic traits are not simple: Lessons from phenylketonuria. Trends in Genetics, 15, 267-272. doi:10.1016/S0168-9525(99)01761-8
- Cooper, D.N., Chen, J., Ball, E.V., Howells, K., Mort, M., Philips, A.D., Chuzhanova, N., Krawczak, M., KehrerSawatzki, H. and Stenson, P.D. (2010) Genes, mutations, and human inherited disease at the dawn of the age of personalized genomics. Human Mutation, 31, 631-655. doi:10.1002/humu.21260

