Advances in Biological Chemistry
Vol.08 No.06(2018), Article ID:88888,20 pages
10.4236/abc.2018.86009
Protective Effects of Extracts, Isolated Compounds from Desmodium uncinatum and Semi-Synthetic Isovitexin Derivatives against Lipid Peroxidation of Hepatocyte’s Membranes
Borice T. Tsafack1, Cyrille L. K. Bomgning2, Jonas Kühlborn3, Romuald T. Fouedjou1, Beaudelaire K. Ponou1, Remy B. Teponno1, Agathe L. Fotio4, Luciano Barboni5, Till Opatz3, Télesphore B. Nguelefack2*, Léon A. Tapondjou1*
1Research Unit of Applied and Environmental Chemistry, Department of Chemistry, Faculty of Science, University of Dschang, Dschang, Cameroon
2Research Unit of Animal Physiology and Phytopharmacology, Department of Animal Biology, Faculty of Science, University of Dschang, Dschang, Cameroon
3Institute of Organic Chemistry, Johannes Gutenberg University Mainz, Mainz, Germany
4Department of Zoology and Animal Physiology, Faculty of Science, University of Buea, Buea, Cameroon
5School of Science and Technology, Chemistry Division, University of Camerino, Camerino, Italy

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 3, 2018; Accepted: November 27, 2018; Published: November 30, 2018
ABSTRACT
Lipid peroxidation plays a pivotal role in the pathogenicity and maintenance of hepatitis. Thus, substances protecting hepatocyte membranes from lipid peroxidation are of great importance in the management of hepatotoxicity and hepatitis. The present work deals with the in vitro hepatoprotective activity of the methanol extract of Desmodium uncinatum, its sub-fractions, the major isolated compounds and some of their semi-synthetic derivatives in order to study structure activity relationships. Using hydrogen peroxide (H2O2)-induced lipid peroxidation of hepatocyte membranes as a model, the hepatoprotective-guided phytochemical survey of the methanol extract of aerial parts of D. uncinatum was carried out by successive column chromatography. One of the most active compounds (Isovitexin) was chemically transformed to yield new semi-synthetic. The identification of isolated and semi-synthetic compounds was performed using NMR techniques, mass spectrometry and by comparison of their data with those reported in the literature. The n-butanol fraction was the most effective (IC50: 22.9 µg/mL) compared to the crude methanol extract (IC50: 43.6 µg/mL) and other fractions. The n-butanol sub-fractions FA (containing non-phenolic compounds) and FB (mainly containing phenolic compounds) exhibited respective IC50 of 14.36 and 128.2 µg/ml. Purification of FA yielded 3-O-β-D-glucopyranosyl-β-sitosterol (1), 3-O-β-D-2-acetyl-amino-2-deoxyglucopyranoxyloleanoic acid (2), (2S, 3S, 4R, 7R, 8Z)-1-O-β-D-glucopyranosyl-2-[(R)-2'-hydroxyarachidoylamino]-docosan-8-ene-3,4,7-triol (4), spiraeamide (5), mannitol (6), while FB afforded essentially three C-glycosylflavonoids namely isovitexin (7), vitexin (8) and vicenin-3 (9). Chemical transformations (methylation, allylation and prenylation) of isovitexin afforded five new semi-synthetic derivatives: 4',5,7-O-trimethylisovitexin (10), 4'-O-allylisovitexin (11), 4',7-O-diallylisovitexin (12), 4'-O-prenylisovitexin (13) and 8-C-prenyl-4',7-O-diprenylisovitexin (14). The screening of these derivatives revealed that allylation did not significantly affect the hepatoprotective activity while methylation, prenylation, number and position of sugar moieties on the A ring of flavonoids significantly reduced it. Results demonstrated that the n-butanol fraction obtained from the methanol extract of Desmdium uncinatum may possess hepatoprotective activity due to its content in C-glycosylflavonoids and cerebrosides. Hydroxyl groups in C-glycosylflavonoids are important for their lipid peroxidation inhibitory activity.
Keywords:
Desmodium uncinatum, Hepatoprotective Activity, Lipid Peroxidation, Hepatocyte Membranes, C-glycosylflavonoids

1. Introduction
The genus Desmodium is a large member of the Papilionaceae (Fabaceae) family. It contains about 350 species mainly distributed in tropical and subtropical regions of the world [1] . Most of Desmodium species are widely used in African traditional medicine and well explored in the treatment of neurological imbalances by the traditional Indian medicinal system [2] . Many of them are known for their hepatoprotective effects [3] . The positive effect of Desmodium adscendens against hepatic infections has been verified in vivo [4] and assigned to its major secondary metabolites including C-glycosylflavonoids identified as vitexin, isovitexin and saponins, mainly soyasaponins [5] . From these results, a number of herbal preparations based on D. adscendens, reputed to have hepatoprotective activity are available on the market.
Currently, lipid peroxidation (LPO) is considered as the main molecular mechanism involved in the oxidative damage of cell structures and in the toxicity process that leads to cell death. In fact, lipid peroxidation is the cornerstone mechanism of the hepatotoxicity exerted by many liver-damaging agents [6] [7] [8] . The major reactive aldehyde resulting from the peroxidation of biological membranes is malondialdehyde (MDA) [9] used as an indicator of tissue damage by a series of chain reactions [10] . Concordantly, in most cases, plant secondary metabolites with antioxidant properties have been found responsible for the hepatoprotective activity of the concerned plants. Indeed, silymarin, a standardized extract of Silybum marianum seeds, is a marketed hepatoprotective medicine containing a mixture of flavonolignans. The primary mechanism of its hepatoprotective effect is the inhibition of cell membrane lipid peroxidation [11] . Moreover, many plant extracts with lipid peroxidation inhibitory effects exhibited hepatoprotective activities [12] [13] . Although there are several methods for measuring the ability of plant extracts to show their hepatoprotective effect by inhibiting lipid peroxidation, the best method remains the monitoring of malondialdehyde, the most studied degradation product of polyunsaturated fatty acids peroxidation [14] .
D. uncinatum is one of the Desmodium species commonly found in the western highlands of Cameroon. It is a large perennial legume with stems that may grow several meters long and trail over surrounding vegetation. Its cylindrical or angular stems are covered with short, hooked hairs that stick to hair or clothing [15] . Previous phytochemical investigations of this plant revealed the presence of flavonoids as uncinanone A, B, C, D and E, uncinacarpan and triterpenoids [16] [17] [18] . Based on the potent hepatoprotective effects of plants from the same genus, we hypothesized that the entire aerial part of D. uncinatum may possess bioactive compounds with hepatoprotective activities resulting from their ability to inhibit lipid peroxidation.
The present study was therefore designed to evaluate the hepatoprotective activitiy of the crude methanolic extract of the aerial part of D. uncinatum and to isolate its bioactive secondary metabolites based on the bioactivity-guided fractionation. Moreover some semi-synthetic derivatives of one of the major active isolated compound (isovitexin) were prepared, tested and the structure activity relationship (SAR) discussed.
2. Materials and Methods
2.1. General Experimental Procedures
1H NMR, 13C NMR, COSY, HMQC and HMBC spectra were performed in deuterated solvents on a Bruker DRX-500 Spectrometer at 500 MHz/125 MHz, on a Bruker AVANCE III-600 MHz/150 Spectrometer, on a varian Mercury plus Spectrometer at 400 MHz/100 MHz and on a Bruker AVANCE 700 spectrometer (Bruker, Germany) at 700 MHz/175 MHz. All chemical shifts (δ) are given in ppm units with reference to tetramethylsilane (TMS) as internal standard and the coupling constants (J) are in Hz. Positive and negative ionisation modes ESI mass spectra were carried out on an Agilent 6210 ESI-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) and on an Agilent Technologies LC/MSD Trap SL (G2445D SL). Column chromatography were performed using 70 - 230 mesh and 230 - 400 mesh silica gel 60 (Merck), and sephadex LH-20. TLC were carried out on precoated silica gel 60 F254 (Merck) plates and spots were visualized by a UV lamp multiband UV-254/365 nm (Model UVGL-58 Upland CA 91786, USA) and by spraying with 50% H2SO4 and heating for 10 min at 110˚C. The structures of all isolated compounds were established by spectroscopic analysis (1D and 2D NMR), ESI mass spectrometry and by comparison of obtained data with those reported in the literature.
2.2. Plant Material
The aerial part of D. uncinatum was collected in Dschang, Menoua Division, Western region of Cameroon in August 2015. The plant material was identified by Professor Zapfack Louis (botanist at the Faculty of Sciences, University of Yaoundé I, Cameroon) at the Cameroon National Herbarium, Yaoundé where a voucher specimen was kept under the reference number HNC/5315.
2.3. Extraction and Bioactivity-Guided Fractionation of Aerial Part of D. uncinatum
Fresh material was cut into small pieces and dried at room temperature for 2 weeks. The powder (4 kg) obtained after grinding was extracted three times (each time for 24 h) with MeOH (15 L) at room temperature before filtration. The combined filtrates were concentrated under reduced pressure to yield the crude MeOH extract (168 g). Part of this crude extract (162 g) was suspended in water (400 mL) and successively extracted with ethyl acetate and n-butanol. The resulting soluble fractions were concentrated to dryness under reduced pressure to give the ethyl acetate fraction (78 g), n-butanol fraction (13 g) and the aqueous residual fraction. The crude extract and its three fractions were kept in the refrigerator at 4˚C until use. After assessing the hepatoprotective activity of the crude extract and its fractions, the active fractions were further submitted to column chromatography separation in order to isolate the possible active compounds (Scheme 1) while the less active fractions were left aside.
The EtOAc fraction was then purified as previously described [18] to yield mainly compounds 1 (23 mg), 2 (12 mg) and 3 (31 mg). Part of the n-butanol fraction (11.5 g) was fractionated over silica gel column chromatography, using a gradient of MeOH in EtOAc ranging from (1:9) to (1:1) to yield five sub-fractions (G1-G5). These sub-fractions were obtained after recombination of fractions of 300 mL based on their TLC profiles. They were separately subjected to Sephadex LH-20 column chromatography using MeOH as eluent to separate non phenolic compounds (fraction A) from phenolics (fraction B). The overall recombination therefore leads to two main fractions, FA (5.5 g) and FB (4 g). Parts of FA and FB were simultaneously submitted to biological screening and chemical investigation. Concerning the chemical investigation, part of fraction A (4.5 g) was purified on a silica gel column eluted with the mixture EtOAc-MeOH-H2O (95-5-2) to yield again compounds 1 (43 mg) and 2 (17 mg) previously obtained from the EtOAc fraction and additionally, compounds 4 (13.4 mg), 5 (10 mg), and 6 (12.5 mg). Part of fraction B (3 g) was repeatedly chromatographed using silica gel column with EtOAc-MeOH-H2O (95-5-2) as eluent to afford compounds 7 (385 mg), 8 (94 mg) and 9 (25 mg).
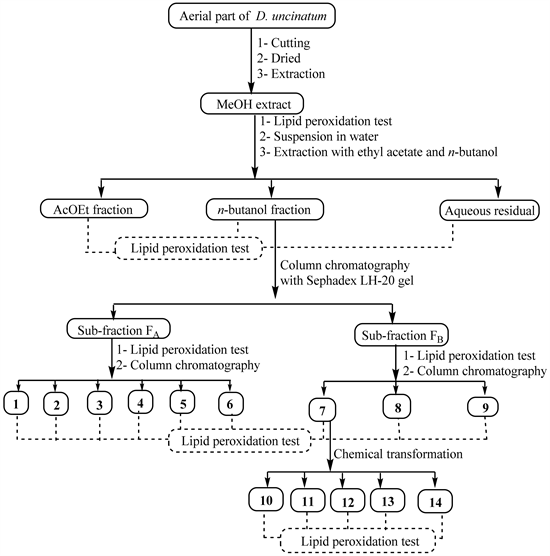
Scheme 1. General work flow indicating extraction, bioguided-fractionation, chemical transformation and pharmacological testing of natural products from the aerial part of D. uncinatum.
2.4. Preparation of Semi-Synthetic Derivatives (10-14) from Isovitexin (7)
2.4.1. Preparation of 4',5,7-O-Trimethylisovitexin (10)
Compound 7 (109 mg) was dissolved in acetone (20 mL); K2CO3 (70.6 mg) and iodomethane (31.4 µL) were successively added to the resulting solution. The mixture was stirred under reflux and monitored by TLC until complete disappearance of the starting material by modifying the method proposed by Li et al. [19] . Afterwards, distilled water was added in order to cool the medium. The cool mixture was extracted with ethyl acetate and the obtained EtOAc phase was washed with water, dried over anhydrous Na2SO4 and the solvent was evaporated in vacuo. The obtained mixture was chromatographed over silica gel using as eluent n-hexane/EtOAc (90:10) to mainly afford compound 10 (23.7 mg, 21.8%).
2.4.2. Preparation of 4'-O-Allylisovitexin (11) and 4',7-O-Allylisovitexin (12)
Compound 7 (118.9 mg) was dissolved in acetone (20 mL) and to this solution were added K2CO3 (40.73 mg) and allyl bromide (25.48 μL) successively. The mixture was magnetically stirred at 25˚C and monitored by TLC until complete disappearance of starting material as previously described by Nkuété et al. [20] and Dong et al. [21] . Using the same treatment procedure as earlier described for compound 10, the reaction mixture obtained was purified using silica gel CC eluted with isocratic solvent system of n-hexane-EtOAc (98:2) that afforded compounds 11 (8.4 mg, 6.4%) and 12 (6.7 mg, 5.15%).
2.4.3. Preparation of 4'-O-Prenylisovitexin (13), and 8-C-Prenyl-4',7-O-Diprenylisovitexin (14)
Isovitexin (7) (133.8 mg) was dissolved in 20 mL of acetone and to the resulting solution were added successively K2CO3 (43.3 mg) and prenyl bromide (36.1 μL). The mixture was magnetically stirred at 25˚C and monitored by TLC until complete disappearance of starting material (12 hours) using the same treatment procedure as earlier described for compound 10, the obtained mixture was thereafter chromatographed over silica gel (230 - 400 mesh) using isocratic solvent system of n-hexane-EtOAc (10:9) as eluent to give compounds 13 (74.3 mg, 47.97%), and 14 (25.4 mg, 12.8%).
2.5. Pharmacological Tests
Biological Materials
The biological materials used for the pharmacological tests were the livers obtained from male Wistar rats, aged between two and three months and weighing 150 to 200 g. They were reared in the animal house of the Laboratory of Animal Physiology and Phytopharmacology at the University of Dschang. Animals were maintained in accordance with the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European Community guidelines (EEC Directive of 1986; 86/609/EEC). They were fed on standard rat food with water ad libitum.
2.6. In Vitro Assessment of D. uncinatum Extracts and Compounds on Lipid Peroxidation of Hepatocytes’s Membranes
Rats were sacrificed by cervical dislocation. The entire liver was rapidly collected and homogenized at 10% in an icy phosphate buffer solution (20 mM; pH: 7.4). The homogenates obtained were centrifuged at 3000 rpm at 4˚C for 10 minutes and the supernatant was discarded. The pellet (200 µL) made of hepatocyte’s membranes were mixed with 200 µL of phosphate buffer (control tubes) or 200 µL of the testing substances (methanol extract, fractions, isolated compounds, semi-synthetic derivatives or silymarin) at the concentrations of 1, 3, 10, 30, 100 or 300 µg/mL. When the testing substance was not water-soluble, the adequate control made of vehicle was conducted. After 10 minutes of incubation at 37˚C, phosphate buffer (200 µL, neutral control) or H2O2 (200 µL, 120 mM, for negative control and testing substances) was added and the mixture incubated for additional 50 minutes. Thereafter, the reaction milieu was thoroughly crushed using an electric tissue grinder. The tubes were then homogenized with Tris buffer (100 µL) and centrifuged at 10,000 rpm for 10 minutes at 4˚C.
To a portion of the supernatant (100 µL), was added a solution of trichloroacetic acid (500 µL, 5%). The mixture was incubated at ambient temperature for 15 minutes and centrifuged at 6000 rpm for 10 minutes at 15˚C. Then, 400 µL of a solution of thiobarbituric acid (1% in 10% of orthophosphoric acid) was added to a portion of the supernatant (400 µL). The mixture was heated at 100˚C for 10 minutes. After cooling, optic densities were spectrophotometrically read at 532 nm to assess the malondialdehyde (MDA) level, indicating of lipid peroxidation. The percentage of inhibition of lipid peroxidation was calculated by the following formular:
2.7. Statistical Analysis
Statistical analysis of the data was performed using Graph Pad prism version 5.01. Data were expressed as mean ± standard error of the mean. The IC50 of each extract was calculated from the non-linear regression curve. To classify tested substances based on the activity, their efficiency index (EI) was calculated with the following formula: EI = Emax/IC50, where Emax is the average of the maximal activity.
3. Results
3.1. In Vitro Effects of D. uncinatum Crude Extract and Its Fractions on Lipid Peroxidation of Hepatocytes’s Membranes
The crude methanol extract of the aerial part of D. uncinatum exhibited a concentration dependent inhibition of lipid peroxidation with an IC50 of 43.6 µg/mL that was lower than that of silymarin (112.2 µg/mL). Although silymarin showed a highest Emax (88.2%), its EI (0.8) was lower than that of the methanol extract (IC50: 43.6 µg/mL, EI: 1.3) (Figure 1).
Fractionation of this crude extract led to three main fractions including the ethyl acetate, n-butanol and residual aqueous fractions which were evaluated. The n-butanol (IC50: 22.9 µg/mL, EI: 3.4) and EtOAc (IC50: 33.8 µg/mL, EI: 1.9) fractions revealed better activity as compared to the crude extract while the residual aqueous fraction was almost inactive (IC50: 370.6 µg/mL, EI: 0.043) (Figure 1). The residual aqueous extract was then kept aside in the further studies.
3.2. Characterisation and Effects of Compounds Isolated from the EtOAc Fraction
Successive chromatography of the EtOAc fraction yielded compounds 1-3 known as 3-O-β-D-glucopyranosyl-β-sitosterol (1), 3-O-β-D-2-acetyl-amino-2-deoxyglucopyranoxyloleanoic acid (2) and hydnocarpin (3) [18] (Figure 2). Compounds 1 and 2 exhibited prooxidant effects on lipid peroxidation by likely increasing the production of MDA by hepatocytes’s membranes whereas compound 3 has relatively no effect, showing a maximal effect of 15.3% (Figure 3).
Figure 1. In vitro effects of D. uncinatum crude extract and its fractions on lipid peroxidation of hepatocytes’s membranes. F-n-BuOH = n-butanol fraction, F-EtOAc = ethyl acetate fraction, F-AR = aqueous residual fraction.
Figure 2. Chemical structures of the isolated compounds (1-9): 1: 3-O-β-D-glucopyranosyl-sitosterol, 2: 3-O-β-D-2-acetyl-amino-2-deoxyglucopyranoxyloleanoic acid, 3: Hydnocarpin, 4: (2S, 3S, 4R, 7R, 8Z)-1-O-D-glucopyranosyl-2-[(R)-2'-hydroxyarachidoylamino]-docosan-8-ene-3,4,7-triol, 5: Spiraeamide, 6: Mannitol, 7: Isovitexin, 8: Vitexin, 9: Vicenin-3.
Figure 3. Inhibitory effect of EtOAc fraction of D. uncinatum and its isolated compounds (1, 2, 3) against hepatocytes’s membrane lipid peroxidation.
3.3. Characterisation and Effects of Compounds Isolated from the n-Butanol Fraction
The n-butanol soluble fraction was separated over a column chromatography into two main sub-fractions FA and FB, which were respectively constituted of non-phenolic and phenolic compounds. These two sub-fractions were also evaluated for their hepatoprotective effect and thereafter subjected to repeated silica gel and sephadex LH-20 column chromatography to afford eight known compounds (1, 2, 4-9). These compounds were respectively identified as: 3-O-β-D-glucosyl-β-sitosterol (1) [22] , 3-O-β-D-2-acetyl-amino-2-deoxyglucopyranoxyl-oleanolic acid (2) [23] , (2S, 3S, 4R, 7R, 8Z)-1-O-β-D-glucopyranosyl-2-[(R)-2'-hydroxyarachidoylamino]-docosan-8-ene-3,4,7-triol (4), Spiraeamide (5) [24] , Mannitol (6) [25] , Isovitexin (7) [26] , vitexin (8) [27] , vicenin 3 (9) [28] (Figure 2).
Sub-fractions FA and FB derived from the n-butanol fraction showed concentration-dependent activities with respective IC50 of 14.3 and 52.3 µg/mL, EI of 3.1 and 1.5 though they were both less effective than the n-butanol fraction itself (IC50: 22.9 µg/mL, EI: 3.4) (Figure 4).
Compounds 4, 5 and 6 isolated from FA, showed a concentration-dependent inhibitory effect over LPO. Compound 4 was the most potent with an IC50 of 22.1 μg/mL and EI of 2.0, followed by compound 5 (IC50: 56.5 μg/mL, EI: 0.5). The least active was mannitol (6). However, the effects of compounds 4, 5 and 6 at concentrations above 10 µg/mL were higher than that of FA (Figure 5).
All the isolated compounds from the phenolic sub-fraction (FB) showed concentration-dependent hepatoprotective activity. Isovitexin (7) and vitexin (8) were more efficient than the sub-fraction B from which they were isolated with respective IC50 of 78.0 and 97.0 µg/mL and EI of 0.8 and 0.7. Compound 9 has a lower inhibitory activity with IC50 of 98.5 µg/mL and EI of 0.2 (Figure 6). It was established from these results that the most active and major compound was isovitexin. Thus it was therefore submitted to further chemical transformations in order to study its structure-activity relationship.
Figure 4. Comparative anti-lipid peroxidative effects of n-BuOH fraction of D. uncinatum and its different sub-fractions (FA and FB) on hepatocyte’s membranes.
Figure 5. Comparative anti-lipid peroxidative effects of sub-fraction FA obtained from the n-butanol fraction and its components on hepatocyte’s membranes.
Figure 6. Comparative anti-lipid peroxidative effects of sub-fraction FB obtained from the n-butanol fraction and its components on hepatocyte’s membranes.
3.4. Characterisation of Semi-Synthetic Derivatives of Isovitexin (7)
The chemical transformations were mainly based on O-alkylation using iodomethane, allyl bromide and prenyl bromide, and this afforded five semi-synthetic flavanone derivatives (Scheme 2) namely: 4',5,7-O-trimethylisovitexin (10), 4'-O-allylisovitexin (11), 4',7-O-diallylisovitexin (12) 4'-O-prenylisovitexin (13) and 8-C-prenyl-4',7-O-diprenylisovitexin (14).
4',5,7-O-trimethylisovitexin (10) was obtained as a yellow powder; = 40.0 (C = 0.98, DMSO); ESIMS: m/z 475.3 [M + H]+ and 497.2 [M + Na]+ (for C24H26O10). 1H NMR (600 MHz, DMSO) and 13C NMR (150 MHz, DMSO) data: See Table 1 and Table 2.
4'-O-allylisovitexin (11) was obtained as yellow powder; = 9.22 (C = 0.6, MeOH); ESIMS (negative mode): m/z 471.2 [M − H]− and 507.1 [M + Cl]− (for C24H24O10). 1H NMR (400 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD) data: See Table 1 and Table 2.

Scheme 2. General procedures used for semi-synthesis of compounds 10-14 from isovitexin: 7: Isovitexin, 10: 4',5,7-O-trimethylisovitexin, 11: 4'-O-allylisovitexin, 12: 4',7-O-diallylisovitexin, 13: 4'-O-prenylisovitexin, 14: 8-C-prenyl-4',7-O-diprenylisovitexin.
Table 1. 1H NMR data of semi-synthetic derivatives (10-14) [δ (ppm), J (Hz)].
*(400 MHz, CD3OD), ** (600 MHz, DMSO).
Table 2. 13C NMR (100 MHz) data of semi-synthetic derivatives (10-14) [δ (ppm)].
*(100 MHz, CD3OD), **(150 MHz, DMSO).
4',7-O-allylisovitexin (12) was obtained as yellow powder; = 4.88 (C = 0.4, MeOH); ESIMS (positive mode): m/z 513.3 [M + H]+ and 535.0 [M + Na]+ (for C27H28O10). 1H NMR (600 MHz, DMSO) and 13C NMR (150 MHz, DMSO) data: See Table 1 and Table 2.
4'-O-prenylisovitexin (13) was obtained as yellow powder; = 0.70 (C = 6.4, MeOH); ESIMS (negative mode): m/z 499.1 [M − H]− (for C26H28O10). 1HNMR (400 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD) data: See Table 1 and Table 2.
8-C-prenyl-4',7-O-diprenylisovitexin (14) was obtained as yellow powder; = 0.70 (C = 6.4, MeOH); ESIMS (negative mode): m/z 671.2 [M + Cl]−, 635.2 [M − H]− (for C36H44O10) and 567.1 [M − Prenyl]−. 1H NMR (400 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD) data: See Table 1 and Table 2.
3.5. Effects of Isovitexin Derivatives (10-14) against Lipid Peroxidation of Hepatocytes’s Membranes
All the semi-synthetic derivatives of isovitexin (7) inhibited lipid peroxidation of hepatocyte’s membranes more or less concentration-dependently (Figure 7). Compound 11 showed an effect similar to that of isovitexin but with IC50 of 167.0 μg/mL and a maximal effect of 54%. Compound 12 exhibited a maximal inhibition of 41% and the inhibitory effect of compound 13 is constant until 100 μg/mL at only 17%. Compound 14 showed a maximal effect of only 24%, with an IC50 of 36.0 µg/mL and EI of 0.6. Compound 10 was found to be the less active among all the tested derivatives with an IC50 of 255.5 μg/mL and an EI of 0.1.
4. Discussion
Oxidative stress, mainly lipid peroxidation (LPO) has been recognised to be involved in the etiology of several liver diseases [29] including viral and non-viral hepatitis [30] . As such, targeting LPO can be a valuable way of managing liver
Figure 7. Comparative inhibitory effect over lipid peroxidation of isovitexin and its semi-synthetic derivatives on hepatocyte’s membranes.
diseases such as hepatitis. Indeed, inhibition of lipid peroxidation is the mechanism of many hepatoptotective drugs, including plant extracts [31] . In a biological system, hydrogen peroxide (H2O2), a reactive oxygen species generated during oxidative stress, is known to cause damage to proteins, nucleic acids, and cell membranes [32] . It is the most effective specie for cellular injury [33] , a well-known oxidant often used as a model compound to induce acute oxidative stress and subsequent lipid peroxidation (LPO) in vitro and in vivo. In the present study, a phytochemical survey of the aerial part of D. uncinatum was conducted through a bioactivity-guided fractionation, using H2O2-induced LPO of hepatocytes’ membranes as model. The crude methanol extract of the plant exhibited a more potent activity against lipid peroxidation as compared to silymarin, a standard commercial drug. Buettner in 1993 [34] explains that antioxidants able to protect lipids from peroxidation can be classified in three main types: 1) preventive antioxidants which hinder reactive oxygen species formation or 2) intercept species responsible of LPO initiation, and 3) chain breaking antioxidant which during the induction process, intercept radical spreading LPO and delays the peroxidation. The plant extract may be acting through one of these mechanisms.
The activity exhibited by the crude methanol extract was however less potent than both its partitioned n-butanol and ethyl acetate fractions while the aqueous residual fraction had no effect. This result suggests that the bioactive molecules of D. uncinatum are n-butanol and ethyl acetate soluble compounds.
Among the compounds isolated from the EtOAc fraction, 1 and 2 were saponins and instead showed prooxidant activity. This result is in contradiction with some of our previous findings which showed the antioxidant activities of some saponins [35] . This lack of activity for compounds 1 and 2 could be attributed to the position and the number of sugars found on the osidic chain branched to the aglycone. Compound 3, a flavolignan with the structure close to that of silibin B the main constituent of silymarin, did not exhibit any activity. This can be explained by the hydrogen bond between the hydroxyl group at position C-5 on the A ring and the carbonyl group at C-4 on the C ring. This hydrogen bond only spares the hydroxyl group at position C-7 on ring A, that has little reactivity. Therefore the absence of hydroxyl group at C-3 position may justify the lack of activity of compound 3.
The cerebrosides enrich sub-fraction (FA) and C-glycosylflavonoids enriched sub-fraction (FB) obtained from the n-butanol fraction were less effective than the n-butanol fraction itself. This suggests that the hepatoprotective effect of the n-BuOH extract might be due to a synergistic action between polar phenolic and non-phenolic compounds present in the whole fraction. Sub-fraction FA was more potent than FB and these findings are somehow surprising as flavonoids are known as reputed antioxidants. The great activity of the cerebrosides (4, 5) which are the main constituents of sub-fraction FA could be attributed to the presence of the alkene functions present on their side chains. Alkene groups can easily prevent or reduce the chain breaking during LPO by providing peroxyl radicals with hydrogen and atoms to stabilize the resulting more stable radicals. Also, the allylic alcohol group found in compound 4 can easily donate hydrogen to stabilize radical HO. produced from the dismutation of H2O2 in order to stop the oxidative process over lipids of the membranes. This could then explain why compound 4 is more active than compound 5 which lacks the allylic alcohol group.
Isovitexin (7) and vitexin (8) isolated from FB showed similar inhibitory effect over LPO and subsequent MDA production contrary to vicenin-3 (9) which was less active. According to their respective EI, the three compounds were less effective than their parent fraction FB, isovitexin being the most potent and vicenin-3 the least potent. The hepatoprotective effects of vitexin and isovitexin reported in this study are in agreement with those previously shown by Manikya et al. [36] using a carbon tetrachloride (CCl4) hepatotoxicity model.
Based on the EI values of the three C-glycoside flavonoids (7, 8 and 9) which have the same aglycon, it could be established that the LPO inhibitory activity depends both on the number of the sugars and their location sites on the aglycon. Isovitexin (7) which had the highest LPO inhibitory and subsequent hepatoprotective activity differed from the other flavonoids (8, 9) by the position of the glucosyl moiety. Thus, the C-glycosylation of flavonoids at C-6 position in the A-ring tends to increase the LPO inhibitory activity than the glycosylation at C-8 position in the same ring. The C-glycosylation at both sites C-6 and C-8 as in compound 9 significantly reduced the activity. These results might be due to steric hindrance imposed by the presence of a sugar moiety at C-8 which can inhibit the proton donation through the hydroxyl group at C-7. In order to verify this hypothesis, five semi-synthetic derivatives of isovitexin (7) were prepared and their activities also evaluated. The combination of the structures and the activities seems to support our proposal.
All the semi-synthetic derivatives of isovitexin (7) namely 10, 11, 12, 13 and 14 inhibited lipid peroxidation of hepatocyte’s membranes more or less in a concentration-dependent manner. It is worth noticing that all semi-synthetic derivatives of isovitexin were less active than isovitexin, thus suggesting that substitution of hydrogens in hydroxyl groups at C-4', C-7 and C-5 by alkyl groups significantly reduced the hepatoprotective effect. It is also clearly established that the activity depends on the nature of the alkyl group present at this position. Although the O-alkylation at C-4' position in B ring reduced the activity, the allyl group (compound 11) has a lower effect on the LPO inhibitory activity of 7 as compared to the prenyl group (compound 13). It can therefore be assumed that the higher the alkyl group is substituted the more the steric hindrance and the less effective the protons of the remaining phenolic hydroxyl groups can be donated thus leading to a reduced activity. Compound 14 was found to be the most efficient, and compounds 12 and 13 the less active. It is also established that compound 12 containing one more allyl group at position C-7 is less active than compound 11. It could therefore be deduced that the activities of C-glycosylflavonoids are mainly attributed to their free hydroxyl groups. This is corroborated with the activity of compound 10 which is drastically reduced compared to that of compound 7.
5. Conclusion
The inhibitory effect of the methanol crude extract of aerial part of D. uncinatum on lipid peroxidation of hepatocyte’s membranes is mainly attributed to compounds of high to medium polarity. These compounds are mainly cerebrosides and C-glycosylflavonoids namely vitexin and isovitexin. From the structure-activity study, it has been established that the number of C-sugars, their sites of fixation on the aglycone and the number of the free hydroxyl groups on the aglycone are capital features for the activity. This study reveals that D. uncinatum possesses potent LPO inhibitory substances that may result to its hepatoprotective activity. It however appears that the n-butanol fraction showed a higher activity as compared to other fractions and to the isolated compounds. Therefore it could be advisable for the development of phytomedicine against hepatitis from D. uncinatum to focus on this particular fraction. The advantage of the present study is that using bio-guided fractionation; we have streamlined the active extract.
Acknowledgements
The authors are grateful to the Alexander von Humboldt Foundation (AvH), Bonn, Germany for the financial support of this work. We thank the Rhineland Platinate Center of Natural Products Research (Mainz, Germany) for funding part of the analytical chemistry involved.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Tsafack, B.T., Bomgning, C.L.K., Kühlborn, J., Fouedjou, R.T., Ponou, B.K., Teponno, R.B., Fotio, A.L., Barboni, L., Opatz, T., Nguelefack, T.B. and Tapondjou, L.A. (2018) Protective Effects of Extracts, Isolated Compounds from Desmodium uncinatum and Semi-Synthetic Isovitexin Derivatives against Lipid Peroxidation of Hepatocyte’s Membranes. Advances in Biological Chemistry, 8, 101-120. https://doi.org/10.4236/abc.2018.86009
References
- 1. Fu, L.G., Taquing, C., Kaiyong, L., Tao, H. and Qi, L. (2008) Higher Plants of China. Qingdao Publishing House. Qingdao
- 2. Ma, X.Q., Zheng, C.J., Hu, C.L., Rahman, K. and Qin, L.P. (2011) The Genus Desmodium (Fabaceae)-Traditional Uses in Chinese Medicine, Phytochemistry and Pharmacology. Journal of Ethnopharmacology, 138, 314-332. https://doi.org/10.1016/j.jep.2011.09.053
- 3. Vedpal, D.S.P., Dhamodaran, P., Chaitnya, M.V.N.L., Duraiswamy, B., Jayaram, U. and Srivastava, N. (2016) Ethnopharmacological and Phytochemical Profile of Three Potent Desmodium Species: Desmodium gangeticum (L.) DC, Desmodium triflorum Linn and Desmodium triquetrum Linn. Journal of Chemical and Pharmaceutical Research, 8, 91-97.
- 4. Tubéry, P., Ragot, J., Lagarde, P., Authier-Derivaux, D., Pidoux, M., Rasolohery, C. and Bourdy, G. (2015) Desmodium adscendens from the Cameroonian Traditional Remedy against Hepatitis to Chemotherapy Support. Hegel, 5, 268-282.
- 5. Pothier, J., Ragot, J. and Galand, N. (2006) Planar Chromatographic Study of Flavonoids and Soyasaponins for Validation of Fingerprints of Desmodium adscendens of Different Origin. Journal of Planar Chromatography-Modern TLC, 19, 191-194.
- 6. Paradis, V., Mathurin, P., Kollinger, M., Imbert-Bismut, F., Charlotte, F., Piton, A., Opolon, P., Holstege, A., Poynard, T. and Bedossa, P. (1997) In Situ Detection of Lipid Peroxidation in Chronic Hepatitis C: Correlation with Pathological Features. Journal of Clinical Pathology, 50, 401-406. https://doi.org/10.1136/jcp.50.5.401
- 7. Kageyama, F., Kobayashi, Y., Kawasaki, T., Toyokuni, S., Uchida, K. and Nakamura, H. (2000) Successful Interferon Therapy Reverses Enhanced Hepatic Iron Accumulation and Lipid Peroxidation in Chronic Hepatitis C. The American Journal of Gastroenterology, 95, 1041-1050. https://doi.org/10.1111/j.1572-0241.2000.01979.x
- 8. Knockaert, L., Berson, A., Ribault, C., Prost, P.E., Fautrel, A., Pajaud, J., Lepage S., Lucas-Clerc, C., Bégué, J.M., Fromenty, B. and Robin, M.A. (2012) Carbon Tetrachloride-Mediated Lipid Peroxidation Induces Early Mitochondrial Alterations in Mouse Liver. Laboratory Investigation, 92, 396-410. https://doi.org/10.1038/labinvest.2011.193
- 9. Vaca, C.E., Wilhelm, J. and Harms-Ringdahl, M. (1988) Interaction of Lipid Peroxidation Products with DNA. A Review. Mutation Research, 195, 137-149. https://doi.org/10.1016/0165-1110(88)90022-X
- 10. Ohkawa, H., Ohishi, N. and Yagi, K. (1979) Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Analytical Biochemistry, 95, 351-358. https://doi.org/10.1016/0003-2697(79)90738-3
- 11. Vargas-Mendoza, N., Madrigal-Santillán, E., Morales-González, á., Esquivel-Soto, J., Esquivel-Chirino, C., y González-Rubio, M.G.L., Gayosso-de-Lucio, J.A. and Morales-González, J.A. (2014) Hepatoprotective Effect of Silymarin. World Journal of Hepatology, 6, 144-149. https://doi.org/10.4254/wjh.v6.i3.144
- 12. Pattanaik, S., Si, S.C., Rout, S.S. and Nayak, S.S. (2013) Evaluation of Hepatoprotective and Lipid Peroxidation Activity of the Leaves of the Plant Crataeva magna Buch Ham (Family Capparidaceae). Der Pharmacia Lettre, 5, 333-337.
- 13. Li, L., Zhou, Y.-F., Li, Y.-L., Wang, L.-L., Arai, H. and Xu, Y. (2017) In Vitro and in Vivo Antioxidative and Hepatoprotective Activity of Aqueous Extract of Cortex dictamni. World Journal of Gastroenterology, 23, 2912-2927. https://doi.org/10.3748/wjg.v23.i16.2912
- 14. Laguerre, M., Lecomte, J. and Villeneuve, P. (2007) Evaluation of the Ability of Antioxidants to Counteract Lipid Oxidation: Existing Methods, New Trends and Challenges. Progress in Lipid Research, 46, 244-282. https://doi.org/10.1016/j.plipres.2007.05.002
- 15. Orwa, C., Mutua, A., Kindt, R., Jamnadass, R. and Simons, A. (2009) Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. 2009.
- 16. Tsanuo, M.K., Hassanali, A., Hooper, A.M., Khan, Z., Kaberia, F., Pickett, J.A. and Wadhams, L.J. (2003) Isoflavanones from the Allelopathic Aqueous Root Exudate of Desmodium uncinatum. Phytochemistry, 64, 265-273. https://doi.org/10.1016/S0031-9422(03)00324-8
- 17. Guchu, S.M., Yenesew, A., Tsanuo, M.K., Gikonyo, N.K., Pickett, J.A., Hooper, A.M. and Hassanali, A. (2007) C-Methylated and C-Prenylated Isoflavonoids from Root Extract of Desmodium uncinatum. Phytochemistry, 68, 646-651. https://doi.org/10.1016/j.phytochem.2006.11.035
- 18. Tsafack, B.T., Ponou, B.K., Teponno, R.B., Nono, R.N., Jenett-Siems, K., Melzig, M.F., Park, H.J. and Tapondjou, L.A. (2017) Integracide K: A New Tetracyclic Triterpenoid from Desmodium uncinatum (Jacq.) DC. (Fabaceae). Natural Product Sciences, 23, 113-118. https://doi.org/10.20307/nps.2017.23.2.113
- 19. Li, N.G., Shi, Z.H., Tang, Y.P., Yang, J.P. and Duan, J.A. (2009) An Efficient Partial Synthesis of 4’-O-Methylquercetin via Regioselective Protection and Alkylation of Quercetin. Beilstein Journal of Organic Chemistry, 5, 1-5.
- 20. Nkuété, A.H.L., Kuete, V., Gozzini, D., Migliolo, L., Oliveira, A.L., Wabo, H.K., Tane, P., Vidari, G., Efferth, T. and Franco, O.L. (2015) Anti-Leukemia Activity of Semi-Synthetic Phenolic Derivatives from Polygonum limbatum Meisn. Chemistry Central Journal, 9, 40. https://doi.org/10.1186/s13065-015-0115-2
- 21. Dong, X., Wang, Y., Liu, T., Wu, P., Gao, J., Xu, J., Yang, B. and Hu, Y. (2011) Flavonoids as Vasorelaxant Agents: Synthesis, Biological Evaluation and Quantitative Structure Activities Relationship (QSAR) Studies. Molecules, 16, 8257-8272. https://doi.org/10.3390/molecules16108257
- 22. Huang, K.F. and Hsu, C.J. (2001) Constituents of Stem Bark of Erythrina arborescens. Journal of Chinese Medicine, 12, 61-67.
- 23. Wang, P., Wang, J., Guo, T. and Li, Y. (2010) Synthesis and Cytotoxic Activity of the N-Acetylglucosamine-Bearing Triterpenoid Saponins. Carbohydrates Research, 345, 607-620. https://doi.org/10.1016/j.carres.2010.01.002
- 24. Mughal, U.R., Mehmood, R., Malik, A., Ali, B., Safder, M. and Tareen, R.B. (2012) Spiraeamide, New Sphingolipid from Spiraea brahuica. Journal of Asian Natural Products Research, 14, 601-606. https://doi.org/10.1080/10286020.2012.672975
- 25. Bock, K., Pedersen, C. and Thogersen, H. (1981) Acid Catalyzed Dehydration of Alditols. Part I. D-glucitol and D-mannitol. Acta Chemica Scandinavica, 35, 441-449. https://doi.org/10.3891/acta.chem.scand.35b-0441
- 26. Peng, J., Fan, G., Hong, Z., Chai, Y. and Wu, Y. (2005) Preparative Separation of Isovitexin and Isoorientin from Patrinia villosa Juss by High-Speed Counter-Current Chromatography. Journal of Chromatography A, 1074, 111-115. https://doi.org/10.1016/j.chroma.2005.03.067
- 27. El-Toumy, S.A., Omara, E.A., Nada, S.A. and Bermejo, J. (2011) Flavone C-Glycosides from Montano abipinnatifida Stems and Evaluation of Hepatoprotective Activity of Extract. Journal of Medicinal Plant Research, 5, 1291-1296.
- 28. Hooper, A.M., Caulfield, J.C., Hao, B., Pickett, J.A., Midega, C.A.O. and Khan, Z.R. (2015) Isolation and Identification of Desmodium Root Exudates from Drought Tolerant Species Used as Intercrops against Strigahermonthica. Phytochemistry, 117, 380-387. https://doi.org/10.1016/j.phytochem.2015.06.026
- 29. Loguercio, C. and Federico, A. (2003) Oxidative Stress in Viral and Alcoholic Hepatitis. Free Radical Biology Medicine, 34, 1-10. https://doi.org/10.1016/S0891-5849(02)01167-X
- 30. Darenskaya, M.A., Grebenkina, L.A., Sholokhov, L.F., Rashidova, M.A., Semenova, N.V., Kolesnikov, S.I. and Kolesnikova, L.I. (2016) Lipid Peroxidation Activity in Women with Chronic Viral Hepatitis. Free Radical Biology and Medicine, 100, S192. https://doi.org/10.1016/j.freeradbiomed.2016.10.525
- 31. Shi, Q.Q., Dang, J., Wen, H.X., Yuan, X., Tao, Y.D. and Wang, Q.L. (2016) Anti-Hepatitis, Antioxidant Activities and Bioactive Compounds of Dracocephalum heterophyllum Extracts. Botanical Studies, 57, 16. https://doi.org/10.1186/s40529-016-0133-y
- 32. Daroui, P., Desai, S.D., Li, T.K., Liu, A.A. and Liu, L.F. (2004) Hydrogen Peroxide Induces Topoisomerase I-Mediated DNA Damage and Cell Death. Journal of Biological Chemistry, 279, 14587-14594. https://doi.org/10.1074/jbc.M311370200
- 33. Rao, N.M., Joshi, N.N., Shinde, S.R., Advani, S.H. and Ghosh, S.N. (1996) Premature Separation of Centromere and Aneuploidy: An Indicator of High Risk in Unaffected Individuals from Familial Breast Cancer Families. European Journal of Cancer Prevention, 5, 343-350. https://doi.org/10.1097/00008469-199610000-00006
- 34. Buettner, G.R. (1993) The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Archives of Biochemistry and Biophysics, 300, 535-543. https://doi.org/10.1006/abbi.1993.1074
- 35. Nguelefack, T.B., Mbakam, F.H.K., Tapondjou, L.A., Watcho, P., Nguelefack-Mbuyo, E.P., Ponou, B.K., Kamanyi, A. and Park, H.J. (2011) A Dimeric Triterpenoid Glycoside and Flavonoid Glycosides with Free Radical-Scavenging Activity Isolated from Rubus rigidus var. Camerunensis. Archives of Pharmacal Research, 34, 543-550. https://doi.org/10.1007/s12272-011-0404-9
- 36. Kumari, M.K., Rao, G.B. and Padmaja, V. (2012) Role of Vitexin and Isovitexin in Hepatoproctective Effect of Alysicarpus monilifer Linn. against CCl4-Induced Hepatotoxicity. Phytopharmacology, 3, 273-285.









