Advances in Biological Chemistry
Vol.3 No.3A(2013), Article ID:33482,5 pages DOI:10.4236/abc.2013.33A001
Protein kinase CK2 in the ER stress response
![]()
Medical Biochemistry and Molecular Biology, Saarland University, Homburg, Germany
Email: *Mathias.Montenarh@uks.eu
Copyright © 2013 Claudia Götz, Mathias Montenarh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 11 April 2013; revised 15 May 2013; accepted 25 May 2013
Keywords: Protein Kinase CK2; ER Stress; Signalling
ABSTRACT
The endoplasmic reticulum is the central organelle within a eukaryotic cell where newly synthesized proteins are processed and properly folded. An excess of unfolded or mis-folded proteins induces ER stress signalling pathways. Usually this means a pro-survival strategy for the cell, whereas under extended stress conditions the ER stress signalling pathways have a pro-apoptotic function. CK2 plays a key role in the regulation of the pro-survival as well as the proapoptotic ER stress signalling by directly modulating the activities of members of the ER stress signalling pathways by phosphorylation, regulating the expression of the key factors of the signalling pathways or binding to regulator proteins. The present review will summarize the state of the art in this new emerging field.
1. INTRODUCTION
The endoplasmic reticulum (ER) of eukaryotic cells is a membrane-enclosed organelle which is closely connected to the nuclear membrane. The ER is responsible for the synthesis, folding, post-translational modification and quality control of numerous secreted and membrane proteins. Folding and maturation of proteins in the ER are influenced by Ca2+ ion concentration in the ER, oxidative stress, hypoxia, energy deprivation, metabolism or protein synthesis. Only properly folded proteins can leave the ER for the Golgi apparatus where they are further processed and finally transported to the plasma membrane whilst mis-folded proteins are directed to degradation. In the case of an accumulation of unfolded or misfolded proteins in the ER, cellular signalling pathways are induced in order to start an adaptive cellular response. This adaptive cellular response leads to an increase in the protein folding capacity of the ER, a reduced global protein biosynthesis and elevated ER-associated protein degradation. In the event of an unsuccessful adaptive cellular response and persistent ER stress, cells would be directed towards autophagy or apoptosis [1,2]. These manifold processes regulating the adaptive cellular response as well as the decision about life and death of a cell must be tightly controlled. Fast and efficient regulation of such cellular processes is achieved mainly by protein kinases, which can regulate key enzymes in these processes by phosphorylation. Among the 518 protein kinases coded by the human genome, protein kinase CK2 plays a key role because life without CK2 is not possible [3-5].
2. PROTEIN KINASE CK2 AND THE ER STRESS RESPONSE
Protein kinase CK2 is a serine/threonine protein kinase composed of two catalytic α- or α’-subunits and two noncatalytic β-subunits. However, there is increasing evidence that the subunits also exist outside of the holoenzymes with functions on their own [6-8]. It is well known that CK2 is implicated in the decision of life and death of a cell [9]. It was shown that CK2 has strong anti-apoptotic properties and inhibition of CK2 activity in many tumour types induces apoptosis [10]. Moreover, UV irradiation increases CK2 dependent phosphorylation of p53 which decreases the pro-apoptotic function of p53 [11]. Beside this UV irradiation stress, heat shock stress has been shown to result in relocalization of CK2 subunits to specific nuclear regions [12]. Stress activating agents like arsenite lead to an activation of CK2 kinase activity by an interaction with p38 MAP kinase [13]. Over the last couple of years there has been increasing evidence that CK2 also plays a key role in ER-stress response. The present review will address specifically the role of CK2 in the ER stress response.
It is known for quite some time that CK2 is located at the endoplasmic reticulum (ER) [14-16] where it phosphorylates ER resident proteins [17-23]. One of the substrates and binding partners for CK2 is HSP90 which is part of a ubiquitously expressed chaperone system in the ER. There, HSP90 is required for the folding, maturation and stabilization of a specific set of target proteins [21- 23]. HSP90 phosphorylation by CK2 is required for the chaperone activity. Binding of HSP90 to CK2 enhances the kinase activity of CK2 and prevents aggregation and inactivation of CK2 [23,24]. The role of CK2 in the chaperone machinery is nicely reviewed by Miyata [25], and is therefore not further addressed in the present review.
In eukaryotic cells there are numerous conditions as well as mutations in specific proteins which result in the accumulation and/or aggregation of unfolded or misfolded proteins in the ER. The ER stress is mediated through three ER transmembrane proteins: the pancreatic ER kinase (PERK), the activating transcription factor-6 (ATF6) or family members such as CREB4, CREBH, and OASIS, and the inositol-requiring enzyme 1 (IRE1) [26]. In the un-stressed cells these transmembrane proteins are maintained in an inactive state through the association with an ER chaperone namely BIP/GRP78 (Figure 1) [20]. Accumulation of unfolded or mis-folded proteins leads to BIP/GRP78 dissociation from and activation of the ER stress mediators. Generally, the ER stress signalling has a pro-survival function by blocking the synthesis of unfolded proteins and removal of unor mis-folded proteins. When the ER stress is too severe and the cell cannot get rid of the ER stress, ER stress signalling switches to pro-apoptotic results. For quite a while it is well accepted that CK2 has a pro-survival function. Inhibition of CK2 leads to apoptosis at least of cancer cells [9,10,27]. Therefore, it is not surprising that CK2 is implicated in the regulation of ER stress signalling.
Although CK2 was found to be associated with the ER [16-19], there was neither a relocalization of CK2 nor an altered expression of the CK2 subunits after ER stress [20]. However, there was an increase in CK2 activity in multiple myeloma cells after ER stress induction by thapsigargin [15].
3. THE PERK MEDIATED PATHWAY
Dissociation of BIP/GRP78 from PERK results in the dimerization of PERK, its autophosphorylation and activation. Active PERK then phosphorylates the eukaryotic initiation factor 2α (eIF2α) which leads to a general inhibition of protein biosynthesis [28]. This inhibition of protein synthesis may help the cell to decrease the load of nascent and unfolded or mis-folded proteins in the ER. In multiple myeloma cells it was shown that CK2α silen-
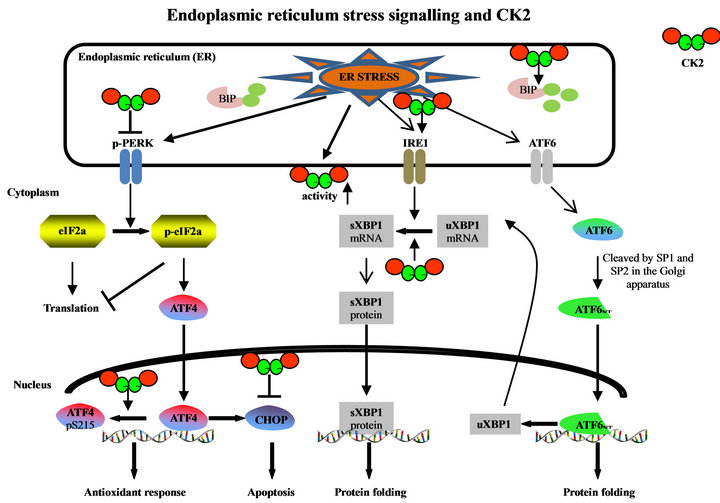
Figure 1. Implication of protein kinase CK2 in ER stress signalling pathways. (uXBP1: unspliced XBP1; sXBP1: spliced XBP1).
cing by miRNA technology causes an increased dephosphorylation and activation of PERK at threonine 981 (Figure 1) [15]. This result suggests that CK2 somehow inhibits PERK signalling but the mechanism of PERK inhibition by CK2 is still an enigma. In the same study it was shown that CK2 inhibition with K27, which is a derivative of the commonly used inhibitor TBB [29], leads to an increase in serine 51 phosphorylation of eIF2α. This increase in the phosphorylation of eIF2α was also shown in another study using HeLa cells and TBB [30] or quinalizarin [31] as CK2 inhibitors [32].
The attenuation of protein biosynthesis by phosphorylated eIF2α is not absolute. Some mRNAs carrying certain regulatory sequences in their 5’ untranslated regions can bypass the translational stop and are translated at even higher rates [33]. One of the mRNAs which is translated in the presence of phosphorylated eIF2α is the one coding for ATF4, which is a member of the CCAAT/enhancer binding protein (C/EBP) family of transcription factors (Figure 1). It was recently shown that inhibition of CK2 leads to an increased transcription of ATF4. Moreover, CK2α binds to ATF4 and it phosphorylates ATF4 at amino acid 215 [32]. Mutant ATF4 which can no longer be phosphorylated by CK2 showed an elevated stability [32]. Also, inhibition of the CK2 enzyme activity leads to an elevated stability of ATF4 [28]. Bimolecular fluorescent complementation experiments revealed that CK2α was bound to nuclear ATF4.
As a transcription factor, ATF4 induces genes which are involved in amino acid metabolism, redox reactions, stress response and protein secretion [33]. In addition to these pro-survival gene products the expression of the pro-apoptosis gene products is regulated by ATF4 [34]. It was shown that CK2 inhibition induces the transcription factor CHOP, which is pro-apoptotic. Transcription of CHOP is mainly regulated by the C/EBP-ATF element in the CHOP promoter which is ATF4 dependent [35]. The induction of CHOP transcription goes along with an elevated level of the CHOP protein [36] at least in hormone sensitive prostate cancer cells.
In contrast to these results with the CHOP promoter CK2 phosphorylation of ATF4 leads to an increase in the expression from the ATF3 promoter as well as the AARE promoter element [32] at least in HeLa cells. Upregulation of CHOP after CK2 inhibition and ER stress signalling seems to be cell type specific because no such induction was observed in hormone refractory prostate cancer cells [36], in glial cells [20] and in multiple myeloma cells [15]. Overexpression of CHOP in hormone refractory prostate cancer cells induces apoptosis [35] indicating that CHOP might play an essential role in apoptosis induction after ER stress. It was already shown that CHOP gene expression in response to ER stress requires an additional factor, which is called NFY. This factor is not only necessary for the constitutive activation of CHOP but it seems to be also an active and essential element for the assembly of transcription factors for CHOP expression after ER stress [37]. A different level of NFY in different cell types may explain that CHOP is not uniformly expressed after CK2 inhibition.
CHOP itself is a target for CK2 i.e. it binds to CK2 and is phosphorylated by CK2. It was shown that CK2 phosphorylation of CHOP inhibits its transcriptional activity [38].
Apoptosis induction as determined by PARP cleavage was also observed in multiple myeloma cells after CK2 inhibition or CK2 silencing although there was no alterations in the CHOP expression indicating that CHOP induction is not absolutely required for apoptosis induction [15].
4. ATF6 MEDIATED ER STRESS SIGNALLING
Dissociation of GRP78 from ATF6 allows the translocation of ATF6 to the Golgi apparatus where it is cleaved into an active transcription factor in the N-terminal part of the molecule (Figure 1). This N-terminal part is then translocated to the nucleus. So far, nothing is known about CK2 targeting ATF6, neither to the ER nor to the Golgi apparatus. Also, nothing is known about an interaction of the other ATF6 family members with CK2 whereas the expression of downstream targets of ATF6 is regulated by CK2. Tunicamycin and thapsigargin are known to induce the expression of BIP/GRP78 and this induction is blocked in cells which were treated with the CK2 inhibitor TBB. The same effect was detected when CK2 expression was knocked down by siRNA experiments [15,20].
5. IRE1 MEDIATED ER-STRESS SIGNALLING
The ER membrane associated IRE1 has two different enzyme activities. The cytoplasmic part of the molecule possesses a serine-threonine kinase domain and the Cterminal part of the molecule harbours an endoribonuclease activity. One of the selective functions of IRE1 is the removal of an intron from the XBP1 mRNA, whose expression is regulated by ATF6. The spliced XBP1 mRNA codes for a transcription factor [39]. Inhibition of CK2 by TBB treatment of glial cells whose ER-stress was induced by tunicamycin or thapsigargin inhibits splicing of XBP1 (Figure 1) [20]. Also in multiple myeloma cells, thapsigargin induces an increase of IRE1 and BIP/GRP78, which was abolished by inhibition of CK2 with K27 [15]. Furthermore, the amount of HSP90 and cdc37 co-precipitating with IRE1 was reduced after CK2 inhibition.
Thus, from these results it is obvious that CK2 is implicated in ER-stress signalling by targeting many different factors within these signalling pathways.
6. CONCLUDING REMARKS
Although most of the knowledge about CK2 and ER stress signalling stems from the PERK/eIF2α/ATF4 pathways, it is still an enigma on how CK2 blocks the activation of PERK under un-stressed conditions. Most of the experiments done so far are performed by pharmacological inhibition of CK2 or by silencing of CK2, whereas, nothing is known about an over-expression of CK2. Does an over-expression of CK2 inhibit ER-stress signalling? Only little is known about the ATF6 and ATF6 family members’ mediated signalling pathway. Are these family members namely CREB4, CREBH and OASIS [40] substrates or binding partners of CK2? Is CK2 somehow implicated in the proteolytic activation of these family members? All experiments described so far are performed with tumour cells. It is an open question whether CK2 also plays a role in ER stress signalling in normal cells.
7. ACKNOWLEDGEMENTS
We thank Nathaniel E.B. Saidu for carefully reading the manuscript.
REFERENCES
- Cao, S.S. and Kaufman, R.J. (2012) Unfolded protein response. Current Biology, 22, R622-R626. doi:10.1016/j.cub.2012.07.004
- Walter, P. and Ron, D. (2011) The unfolded protein response: From stress pathway to homeostatic regulation. Science, 334, 1081-1086. doi:10.1126/science.1209038
- Dominguez, I., Degano, I.R., Chea, K., et al. (2011) CK2- alpha is essential for embryonic morphogenesis. Molecular and Cellular Biochemistry, 356, 209-216. doi:10.1007/s11010-011-0961-8
- Lou, D.Y., Dominguez, I., Toselli, P., et al. (2007) The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Molecular and Cellular Biochemistry, 28, 131-139.
- Buchou, T., Vernet, M., Blond, O., et al. (2003) Disruption of the regulatory b subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Molecular and Cellular Biochemistry, 23, 908-915. doi:10.1128/MCB.23.3.908-915.2003
- Lüscher, B. and Litchfield, D.W. (1994) Biosynthesis of casein kinase II in lymphoid cell lines. European Journal of Biochemistry, 220, 521-526. doi:10.1111/j.1432-1033.1994.tb18651.x
- Guerra, B., Siemer, S., Boldyreff, B., et al. (1999) Protein kinase CK2: Evidence for a protein kinase CK2b subunit fraction, devoid of the catalytic CK2a subunit, in mouse brain and testicles. FEBS Letters, 462, 353-357. doi:10.1016/S0014-5793(99)01553-7
- Stalter, G., Siemer, S., Becht, E., et al. (1994) Asymmetric expression of protein kinase CK2 in human kidney tumors. Biochemical and Biophysical Research Communications, 202, 141-147. doi:10.1006/bbrc.1994.1904
- Gabriel, M. and Litchfield, D.W. (2013) Protein kinase CK2: At the crossroad of pathways controlling cell proliferation and survival. In: Pinna, L.A., Ed., Protein Kinase CK2, John Wiley & Sons, Inc., Hoboken, 169- 189.
- Trembley, J.H., Wu, J., Unger, G.M., et al. (2013) CK2 suppression of apoptosis and its implication in cancer biology and therapy. In: Pinna, L.A., Ed., Protein Kinase CK2, John Wiley & Sons, Inc., Hoboken, 319-343.
- Blaydes, J.P. and Hupp, T.R. (1998) DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site. Oncogene, 17, 1045-1052. doi:10.1038/sj.onc.1202014
- Gerber, D.A., Souquere-Besse, S., Puvion, F., et al. (2000) Heat-induced relocalization of protein kinase CK2—Implication of CK2 in the context of cellular stress. Journal of Biological Chemistry, 275, 23919-23926. doi:10.1074/jbc.M002697200
- Sayed, M., Kim, S.O., Salh, B.S., et al. (2000) Stressinduced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. Journal of Biological Chemistry, 275, 16569-16573. doi:10.1074/jbc.M000312200
- Yamane, K. and Kinsella, T.J. (2005) CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Research, 65, 4362-4367. doi:10.1158/0008-5472.CAN-04-3941
- Manni, S., Brancalion, A., Quotti, T.L., et al. (2012) Protein kinase CK2 protects multiple myeloma cells from ER stress-induced apoptosis and from the cytotoxic effect of HSP90 inhibition through regulation of the unfolded protein response. Clinical Cancer Research, 18, 1888-1900. doi:10.1158/1078-0432.CCR-11-1789
- Faust, M., Jung, M., Günther, J., et al. (2001) Localization of individual subunits of protein kinase CK2 to the endoplasmic reticulum and to the Golgi apparatus. Molecular and Cellular Biochemistry, 227, 73-80. doi:10.1023/A:1013129410551
- Gruss, O.J., Feick, P., Frank, R., et al. (1999) Phosphorylation of components of the ER translocation site. European Journal of Biochemistry, 260, 785-793. doi:10.1046/j.1432-1327.1999.00215.x
- Götz, C., Müller, A., Montenarh, M., et al. (2009) The ER-membrane-resident Hsp40 ERj1 is a novel substrate for protein kinase CK2. Biochemical and Biophysical Research Communications, 388, 637-642. doi:10.1016/j.bbrc.2009.07.146
- Ampofo, E., Welker, S., Jung, M., et al. (2013) CK2 phosphorylation of human Sec63 regulates its interaction with Sec62. Biochimica et Biophysica Acta, 1830, 2938-2945. doi:10.1016/j.bbagen.2012.12.020
- Hosoi, T., Korematsu, K., Horie, N., et al. (2012) Inhibition of casein kinase 2 modulates XBP1-GRP78 arm of unfolded protein responses in cultured glial cells. PLoS. ONE, 7, e40144. doi:10.1371/journal.pone.0040144
- Meggio, F., Agostinis, P. and Pinna, L.A. (1985) Casein kinases and their protein substrates in rat liver cytosol: evidence for their participation in multimolecular systems. Biochimica et Biophysica Acta, 846, 248-256. doi:10.1016/0167-4889(85)90072-2
- Dougherty, J.J., Rabideau, D.A., Iannotti, A.M., et al. (1987) Identification of the 90 kDa substrate of rat liver type II casein kinase with the heat shock protein which binds steroid receptors. Biochimica et Biophysica Acta, 927, 74-80. doi:10.1016/0167-4889(87)90067-X
- Miyata, Y. and Yahara, I. (1995) Interaction between casein kinase II and the 90-kDa stress protein, HSP90. Biochemistry, 34, 8123-8129. doi:10.1021/bi00025a019
- Miyata, Y. and Yahara, I. (2002) The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. Journal of Biological Chemistry, 267, 7042-7047.
- Miyata, Y. (2013) The pivotal role of CK2 in the kinome-targeting Hsp90 chaperone machinery. In: Pinna, L.A. Ed., Protein Kinase CK2, John Wiley & Sons, Inc., Hoboken, 205-238.
- Gorman, A.M., Healy, S.J., Jager, R., et al. (2012) Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacology and Therpeutics, 134, 306-316. doi:10.1016/j.pharmthera.2012.02.003
- Guerra, B. and Issinger, O.-G. (2013) CK2: A global regulator of cell survival. In: Pinna, L.A. Ed., Protein Kinase CK2, John Wiley & Sons, Inc., Hoboken, 239- 266.
- Harding, H.P., Zhang, Y. and Ron, D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 397, 271-274. doi:10.1038/16729
- Niefind, K. and Battistutta, R. (2013) Structural bases protein kinase CK2 function and inhibition. In: Pinna, L.A. Ed., Protein Kinase CK2, John Wiley & Sons, Inc., Hoboken, 3-75.
- Sarno, S., Reddy, H., Meggio, F., et al. (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (casein kinase-2’). FEBS Letters, 496, 44-48. doi:10.1016/S0014-5793(01)02404-8
- Cozza, G., Mazzorana, M., Papinutto, E., et al. (2009) Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochemical Journal, 421, 387-395. doi:10.1042/BJ20090069
- Ampofo, E., Sokolowsky, T., Götz, C., et al. (2013) Functional interaction of protein kinase CK2 and activating transcription factor 4 (ATF4), a key player in the cellular stress response. Biochimica et Biophysica Acta: Molecular Cell Research, 1833, 439-451.
- Schroder, M. and Kaufman, R.J. (2005) The mammalian unfolded protein response. Annual Reviews in Biochemistry, 74, 739-789.
- Harding, H.P., Novoa, I., Zhang, Y., et al. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Molecular Cell, 6, 1099- 1108. doi:10.1016/S1097-2765(00)00108-8
- Schneider, C.C., Ampofo, E. and Montenarh, M. (2012) CK2 regulates ATF4 and CHOP transcription within the cellular stress response signalling pathway. Cell Signalling, 24, 1797-1802.
- Hessenauer, A., Schneider, C.C., Götz, C., et al. (2011) CK2 inhibition induces apoptosis via the ER stress response. Cell Signalling, 23, 145-151. doi:10.1016/j.cellsig.2010.08.014
- Ubeda, M. and Habener, J.F. (2000) CHOP gene expression in response to endoplasmic-reticular stress requires NFY interaction with different domains of a conserved DNA-binding element. Nucleic Acids Research, 28, 4987- 4997. doi:10.1093/nar/28.24.4987
- Ubeda, M. and Habener, J.F. (2003) CHOP transcription factor phosphorylation by casein kinase 2 inhibits transcriptional activation. Journal of Biological Chemistry, 278, 40514-40520. doi:10.1074/jbc.M306404200
- Yoshida, H., Matsui, T., Yamamoto, A., et al. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107, 881-891. doi:10.1016/S0092-8674(01)00611-0
- Asada, R., Kanemoto, S., Kondo, S., et al. (2011) The signaling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. Journal of Biochemistry, 149, 507-518. doi:10.1093/jb/mvr041
NOTES
*Corresponding author.

