Advances in Biological Chemistry
Vol.3 No.2(2013), Article ID:30748,5 pages DOI:10.4236/abc.2013.32020
Physiopathology of parotid cell energetics
![]()
Laboratory of Experimental Hormonology, Université Libre de Bruxelles, Brussels, Belgium
Email: *malaisse@ulb.ac.be
Copyright © 2013 Cédric Jurysta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 10 January 2013; revised 7 March 2013; accepted 14 April 2013
Keywords: D-Glucose Metabolism; Rat Parotid Cells
ABSTRACT
Attention was recently drawn to the production of Dglucose by salivary glands and its concentration in the saliva of both normal and diabetic subjects. Likewise, the identity and expression of selected transporters involved in the handling of the hexose by salivary glands were recently investigated in both normal and diabetic rats. In consideration of this information, the present report aims at providing an integrated review on the physiopathology of parotid cell energetics.
1. INTRODUCTION
The production of D-glucose by salivary glands and the concentration of the hexose in saliva are both higher in diabetic patients than in control subjects [1]. Several transporters including GLUT1, GLUT2, GLUT4 and SGLT1 were recently considered as possibly playing a role in the handling of D-glucose in salivary glands [2,3]. The expression of GLUT4 documented in the parotid gland and submaxillary gland of both control and diabetic rats may also be relevant to the regulation of Salivary cell energetics. In such a perspective, the present report aims at providing an integrated review on the physiopathology of rat parotid cell energetics.
Emphasis is placed on the several methods that can be used and were indeed used to assess such variables as the overall energy status judged by the uptake of Tc-MIBI, the uptake of selected hexoses and heptoses in order to investigate the possible role of distinct sugar transporters, the phosphorylation of these carbohydrates in cell homogenates in order to identify the concerned catalytic hexokinases(s), the utilization and oxidation of distinct simple sugars and their reciprocal metabolic effects, the eventual intracellular accumulation of glycogen in situations of sustained hyperglycemia, the participation of a mitochondrial carbonic anhydrase in the conversion of carbon dioxide to bicarbonate anions, the activity and secretion of amylase, and the environmental modulation of selected biochemical variables.
2. 99 mTc-sesta-(2-methoxy-isobutyl-isonitrile) (Tc-MIBI) UPTAKE
It was proposed by Blocklet et al. [4] that the uptake of Tc-MIBI by parotid cells may be useful to detect alteration of nutrient metabolism, e.g. in cells deprived of any exogenous nutrient. After 60 min incubation at 37˚C in the presence of 16.7 mM D-glucose, the net uptake of Tc-MIBI (44 nM) by rat parotid cells averaged 5.92 ± 0.38 fmol/103 cells (n = 20). Over 2 to 90 min incubation, the time course for Tc-MIBI uptake indicates a progressive increase towards an equilibrium value. Over a 60 min-incubation, such an uptake was virtually proportional to the concentration of Tc-MIBI (20 - 100 nM). Over the same incubation time, a concentration of 5.6 mM D-glucose was sufficient to increase Tc-MIBI uptake above basal value to a maximal level, no further increase in uptake being observed when the concentration of the hexose was raised to 16.7 mM [4]. Salivary glands also exhibit a high uptake of Tc-MIBI in clinical studies [Blocklet, D. and Schoutens, A., unpublished observation].
3. UPTAKE OF D-GLUCOSE AND D-MANNOHEPTULOSE
The intracellular accumulation of 3-O-methyl-D-[U-14C] glucose (4 - 6 µM) was judged from the total 3-Omethyl-D-[U-14C]glucose (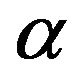 ) and 3HOH (
) and 3HOH ( ) spaces and from the total [U-14C]glucose (
) spaces and from the total [U-14C]glucose ( ) and 3HOH (
) and 3HOH ( ) spaces, according to the following expression
) spaces, according to the following expression
 .
.
The intracellular distribution space of 3-O-methyl-D- [U-14C]glucose reached its equilibrium value, averaging 26.8% ± 1.4% of the intracellular 3HOH space, within 10 min of incubation at 37˚C. In the light of these findings, all further experiments were conducted over 30 min incubation at 37˚C. Incidentally, the distribution space of [U-14C]sucrose (1.0 mM) was not significantly different from that of L-[1-14C]glucose (also 1.0 mM) [5].
The intracellular space of D-[U-14C]glucose (8.3 mM), calculated as indicated above and expressed relative to the intracellular 3HOH space, averaged 23.9% ± 4.2%, a value not significantly different from that found with 3-O-methyl-D-[U-14C]glucose.
In order to investigate the possible role of GLUT2 in the uptake of carbohydrates, experiments were also conducted in the presence of D-[3H]mannoheptulose (0.1 mM) and D-[3H]mannoheptulose hexacetate (also 0.1 mM). Relative to the intracellular 3HOH space, that of D-mannoheptulose did not exceed 13.5% ± 1.1%, as compared to 248.7% ± 15.7% for its hexacetate ester. Comparable results were obtained with D-[1-14C] mannoheptulose (0.1 mM), in which case the intracellular space of the heptose averaged 13.3% ± 2.3% of the intracellular 3HOH space [5].
4. PHOSPHORYLATION OF D-GLUCOSE AND D-MANNOHEPTULOSE
In a first study, the phosphorylation of D-glucose (0.1 to 20.0 mM) by rat parotid gland homogenates, at increasing concentrations of D-mannoheptulose (1.0 to 20.0 mM), yielded reaction velocities indicative of competitive inhibition of hexokinase by the heptose. Thus, in a Dixon plot, the regression lines obtained at the four concentrations of D-glucose had a common intercept in the left quadrant, corresponding to a Ki close to 2.2 mM Dmannoheptulose [6].
In a further study, the phosphorylation of D-[U- 14C]glucose (0.1 to 3.0 mM) by rat parotid gland homogenates yielded a Km of 0.09 mM, close to that found in the same study with beef heart hexokinase, i.e. 0.11 mM. D-mannoheptulose (3.0 mM) inhibited in a competitive manner the phosphorylation of D-glucose [7]. D-mannoheptulose hexaacetate (0.3 to 3.0 mM) did not inhibit the phosphorylation of D-glucose at any of the four concentrations of the hexose used in these experiments (0.1 mM, 0.3 mM, 1.0 mM and 3.0 mM).
The phosphorylation of D-glucose (16.7 mM) by rat parotid gland homogenates was unaffected by D-fructose (80.0 mM) and decreased by no more than 2.8% to 4.5% by 3-O-methyl-D-glucose and D-galactose, respectively, also tested at a 80.0 mM concentration [8].
Rat parotid gland homogenates catalyzed the phosphorylation of D-[3H]mannoheptulose (0.1 mM) at a rate not exceeding 4.83 ± 0.32 pmol/min per mg wet weight, i.e. a value representing no more than 1.2‰ ± 0.2 ‰ of the paired value found with D-[U-14C]glucose (10.0 mM). D-glucose (10.0 mM) decreased the phosphorylation of the tritiated heptose to 1.5% ± 0.4% of the paired control value (no glucose) recorded in the parotid gland homogenates [9].
5. D-GLUCOSE AND D-MANNOHEPTULOSE METABOLISM
D-[U-14C]glucose oxidation by intact rat parotid cells increases from 4.71 ± 0.26 to 6.31 ± 0.11 and 7.04 ± 0.17 pmol/103 cells per 60 min as the concentration of the hexose is raised from 1.7 to 2.8 and 16.7 mM [6]. In the 1.0 to 10.0 mM concentration range, D-mannoheptulose fails to affect adversely the oxidation of D-[U-14C] glucose (1.7 mM, 2.8 mM or 16.7 mM).
D-mannoheptulose (10.0 mM) also fails to affect adversely the generation of 3HOH from D-[5-3H]glucose and that of 14C-labeled acidic metabolites and amino acids from D-[U-14C]glucose in rat parotid cells incubated for 120 min in the presence of 5.6 mM D-glucose. In sharp contrast to these findings, the hexaacetate of D-mannoheptulose (0.1 to 4.8 mM) causes, under the same experimental conditions, a concentration-related decrease in D-[5-3H]glucose utilization and D-[U-14C] glucose conversion to 14CO2 and 14C-labeled amino acid or acidic metabolites [7]. Control experiments indicated that neither acetate nor methylacetate, tested at a concentration (6.0 mM) equimolar to that of acetate residues of 1.0 mM D-mannoheptulose hexaacetate reproduced the inhibitory action of the latter ester on D-glucose metabolism.
Like D-mannoheptulose (1.0 mM), 3-O-methyl-D-glucose (80.0 mM) does not inhibit either D-[5-3H]glucose utilization and D-[U-14C]glucose oxidation by rat parotid cells incubated for 60 min in the presence of 16.7 mM D-glucose [8].
No generation of 3HOH from D-[3H]mannoheptulose (0.1 mM) and no generation of 14CO2 from D-[1-14C] mannoheptulose (0.1 mM) could be detected in rat parotid cells incubated for 30 min at 37˚C in the presence of the heptose [5].
In a further study, the generation of 3HOH from D-[3-3H]glucose by intact rat parotid cells incubated for 120 min in the presence of 10.0 mM D-glucose was found to underestimate the utilization of the hexose. Thus, the generation of 3HOH from D-[3-3H]glucose only represented 83.6% ± 1.5% of the mean corresponding value recorded in the presence of D-[5-3H]glucose, such a difference being apparently attributable to a partial escape from detritiation of the enantiomer of [1- 3H]glycerone 3-phosphate generated from D-[3-3H] glucose [10].
6. D-FRUCTOSE PHOSPHORYLATION AND METABOLISM
As judged from the rate of D-fructose (1.0 mM) phosphorylation by homogenates first heated for 5 min at 70˚C, the activity of fructokinase in rat parotid glands is close to the limit of detection, in sharp contrast to the findings recorded within the same study in rat liver homogenates [11].
In homogenates of rat parotid glands, D-fructose (10.0 mM) did not affect significantly the phosphorylation of D-[U-14C]glucose (10.0 mM). D-glucose (10.0 mM), however, suppressed the phosphorylation of D-[U-14C] fructose (10.0 mM), half-maximal inhibition being recorded at a concentration of D-glucose slightly above 0.3 mM. D-glucose (0.3 to 30.0 mM) also caused a severe decrease of D-[5-3H]fructose utilization, D-[U-14C] fructose oxidation and the oxidation/utilization ratio of the ketohexose, whether in the presence of 1.0 or 20.0 mM D-fructose [12].
In the absence of D-glucose, a rise in D-fructose concentration from 1.0 to 20.0 mM increased to virtually the same relative extent, i.e. about a 16-fold increase, the generation of 3HOH from D-[5-3H]fructose and that of 14CO2 from D-[U-14C]glucose. The generation of 14Clabeled amino acids and acidic metabolites from D- [U-14C]fructose was affected by a change in the concentration of the ketohexose and by D-glucose (1.0 or 20.0 mM) in a manner comparable to that observed for the oxidation of D-[U-14C]fructose. As judged from the paired ratio between either D-[1-14C]fructose or D-[6-14C] fructose oxidation and D-[5-3H]fructose utilization, the fraction of D-fructose metabolized to CO2 and Dglyceraldehyde 3-phosphate via the pentose shunt averaged 4.68% ± 0.51% in the sole presence of the ketohexose (10.0 mM) and was decreased to 2.30% ± 0.50% in the concomitant presence of D-fructose (10.0 mM) and D-glucose (also 10.0 mM) [12].
These findings were confirmer in a further study, in which it was documented that the generation of 14CO2, radioactive amino acids and 14C-labeled acidic metabolites from D-[U-14C]fructose (10.0 mM), whether measured in parotid cells incubated in the absence or presence of D-glucose (10.0 mM), was essentially similar in cells obtained from control rats or hereditarily diabetic GotoKakizaki rats [13].
7. GLYCOGEN ACCUMULATION
In situations of sustained hyperglycemia, glycogen accumulates to a larger extent in insulin-producing  - cells than in acinar cells of the pancreas. In three series of investigations, it was investigated whether parotid glands also accumulate glycogen in comparable situations.
- cells than in acinar cells of the pancreas. In three series of investigations, it was investigated whether parotid glands also accumulate glycogen in comparable situations.
In the first study, normal rats were infused for 48 hours with a hypertonic solution of D-glucose (1.67 M) containing a tracer amount of D-[U-14C]glucose and delivered at an infusion rate of 2.8 ml/60 min per rat. The radioactive content of the parotid gland averaged, when expressed relative to the paired blood value 2.46 ± 0.29 µL/mg, a value somewhat higher, albeit not significantly so, than that found in the pancreas (1.39 ± 0.36 µL/mg) and much lower than that found in the liver. A comparable hierarchy was observed for the radioactive glycogen content of these 3 organs. The radioactive glycogen content of the parotid gland, expressed as D-glucose equivalent, averaged 5.10 ± 1.02 nmol/mg. These findings document that sustained hyperglycemia may result in a sizeable de novo accumulation of glycogen in parotid glands [14].
In a somewhat comparable perspective, control rats, rats rendered diabetic by a prior administration of streptozotocin and streptozotocin-injected rats treated with exogenous insulin were injected intravenously at time zero with 2-deoxy-2-[18F]fluoro-D-glucose [15]. Whilst the radioactive content (cpm/mg) of the parotid gland merely underwent a modest increase between the 3rd and 240th min after the latter injection, whether in control or diabetic animals treated with insulin, the parotid/plasma radioactive ratio was one order of magnitude higher at min 240 than at earlier times (min 3 and min 15). Whether 3 min, 15 min or 240 min after the same injection, the radioactive content (cpm/mg wet weight) of the parotid gland of control rats was somewhat higher than that of the pancreatic gland. As a rule, it failed to differ significantly in control and either untreated or insulintreated streptozotocin-induced diabetic rats. Thus, whether at min 3, min 15 or min 240, the parotid/plasma radioactive ratio averaged in the insulin-treated diabetic rats 132.0% ± 14.6% (n = 12) of the mean corresponding values found at the same time in control animals (100.0% ± 9.0%; n = 13).
In the last study, untreated streptozotocin-induced diabetic rats and control rats infused with a hypertonic solution of D-glucose (16.7 mM) in order to raise their plasma D-glucose concentration from an initial value 6.81 ± 0.29 mM to a value of 33.28 ± 6.54 mM virtually identical to that found in the diabetic animals (34.90 ± 2.82 mM) were examined 8 hours after the intravenous injection of 2-deoxy-2-[18F]fluoro-D-glucose. Once again, the parotid/blood radioactive ratio was somewhat higher than the pancreatic/blood radioactive ratio, whether in control or STZ rats, and somewhat higher in diabetic rats than in control animals [16]. The latter finding contrasted with a much lower muscle/blood radioactive ratio in the diabetic rats than in the control animals infused with the hypertonic solution of D-glucose.
8. CARBONIC ANHYDRASE ACTIVITY, AMYLASE ACTIVITY AND AMYLASE SECRETION
As judged from the conversion of 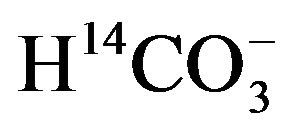 to 14CO2, the activity of carbonic anhydrase in rat parotid cell homogenates is virtually proportional to
to 14CO2, the activity of carbonic anhydrase in rat parotid cell homogenates is virtually proportional to 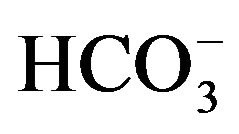 concentration, increasing from 1.11 ± 0.04 (n = 4) to 11.68 ± 3.01 (n = 8) nmol/min per mg protein as the concentration of
concentration, increasing from 1.11 ± 0.04 (n = 4) to 11.68 ± 3.01 (n = 8) nmol/min per mg protein as the concentration of 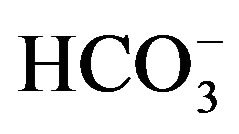 is raised from 50 µM to 0.5 mM [17]. In the presence of 50 µM
is raised from 50 µM to 0.5 mM [17]. In the presence of 50 µM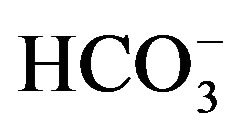 , the activity of the enzyme, expressed per milligram of protein, is four to five times higher in parotid cell homogenates than in islet homogenates of the same animals. In the parotid cell homogenates, the activity of carbonic anhydrase, as measured in the presence of 50 µM
, the activity of the enzyme, expressed per milligram of protein, is four to five times higher in parotid cell homogenates than in islet homogenates of the same animals. In the parotid cell homogenates, the activity of carbonic anhydrase, as measured in the presence of 50 µM , is inhibited by acetazolamide, as well as by hydrochlorothiazide, with respective inhibition constant values close to 2.2 and 27.8 µM [17]. No significant difference in carbonic anhydrase activity, as measured in the presence of 0.5 mM
, is inhibited by acetazolamide, as well as by hydrochlorothiazide, with respective inhibition constant values close to 2.2 and 27.8 µM [17]. No significant difference in carbonic anhydrase activity, as measured in the presence of 0.5 mM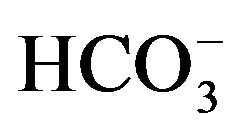 , was observed when comparing female Wistar and Goto-Kakizaki rats of comparable age, whether the results were expressed per mg protein or µg DNA [18].
, was observed when comparing female Wistar and Goto-Kakizaki rats of comparable age, whether the results were expressed per mg protein or µg DNA [18].
The activity of amylase in the homogenates of parotid cells also failed to differ significantly in Wistar and Goto-Kakizaki rats, with mean respective values of 13.4 ± 1.3 (n = 8) and 14.8 ± 1.1 (n = 12) µU/mg protein [18].
When rat parotid cells are incubated for 30 min at 37˚C in salt-balanced media containing either 102 mM NaCl and 24 mM NaHCO3 or 126 mM NaCl and no bicarbonate, the output of a-amylase is decreased in the absence of bicarbonate to 50.1% ± 5.5% (basal output), 48.9% ± 4.4% (carbamylcholine-stimulated output) and 50.3% ± 4.7% (isoproterenol-stimulated output) of the mean corresponding values found within the same experiment(s) in the presence of bicarbonate (n = 15 in all cases). It was proposed, therefore, that the 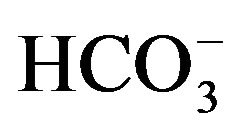 anion participates in the secretory sequence at sites distal to the identification of secretagogues [19].
anion participates in the secretory sequence at sites distal to the identification of secretagogues [19].
9. ENVIRONMENTAL MODULATION OF BIOCHEMICAL VARIABLES
In the preceding section of this review, it was already underlined that hereditary diabetes in Goto-Kakizaki rats fails to affect either the activity of carbonic anhydrase or the amylase content of parotid cells. It should not be ignored, however, that, in salivary glands, selected biochemical variables may be affected by environmental factors.
For instance, the total fatty acid content of phospholipids in the submandibular gland, expressed relative to tissue wet weight, is almost twice higher in young rats (8 - 9 weeks) than in older animals (22 - 23 weeks), this coinciding with lower C16:0/C16:1w7, C18:0/C18:1w9, C20:3w6/C20:4w6 and C22:5w3/C22:6w3 ratios in the older rats than in the young animals [20]. Likewise the total fatty acid content of triglycerides, always expressed relative to tissue wet weight, is about twice higher in old rats than in young rats, with a significant inverse correlation between the individual values for the weight percentage of C20:4w6 and total fatty acid content of submandibular gland triglycerides.
Likewise, severe alterations of the phospholipid and triglyceride fatty acid pattern were observed in the submandibular gland of rats depleted in long-chain polyunsaturated w3 fatty acids [20]. Injection of these rats with a medium-chain triglyceride:fish oil emulsion 60 - 120 min before sacrifice increased the phospholipid C22:5w3 and C22:6w3 content of the submandibular gland [20, 21].
10. CONCLUSION
The present review draws attention to a number of biochemical features relative to the energetics of salivary glands, especially parotid cells. In our opinion, this information should not be ignored when considering the perturbation of salivary gland metabolism and function, such as that encountered in diabetic patients. More precisely, the present review deals with the relevant methods to assess possible alterations of metabolic events in selected steps of carbohydrate transport, phosphorylation and further anabolic or catabolic processes.
REFERENCES
- Jurysta, C., Bulur, N., Oguzhan, B., Satman, I., Yilmaz, T.M., Malaisse, W.J. and Sener, A. (2009) Salivary glucose concentration and excretion in normal and diabetic subjects. Journal of Biomedicine and Biotechnology, 2009, 1-16.
- Jurysta, C., Nicaise, C., Cetik, S., Louchami, K., Malaisse, W.J. and Sener, A. (2012) Glucose transport by acinar cells in rat parotid glands. Cellular Physiology and Biochemistry, 29, 325-330. doi:10.1159/000338487
- Jurysta, C., Nicaise, C., Giroix, M.-H., Cetik, S., Malaisse, W.J. and Sener, A. (2013) Comparison of GLUT1, GLUT2, GLUT4 and SGLT1 mRNA-expression in the salivary glands and six other organs of control, streptozotocin-induced and Goto-Kakizaki diabetic rats. Cellular Physiology and Biochemistry, 31, 34-43. doi:10.1159/000343347
- Blocklet, D., Jijakli, H., Sener, A., Schoutens, A. and Malaisse, W.J. (1998) 99 mTc-sesta-(2-methoxy-isobutylisonitrile) uptake by pancreatic islets, parotid cells, and mammary carcinoma cells. Endocrine, 9, 113-117. doi:10.1385/ENDO:9:1:113
- Ramirez, R., Courtois, P., Ladrière, L., Kadiata, M.M., Sener, A. and Malaisse, W.J. (2001) Uptake of D-mannoheptulose by rat erythrocytes, hepatocytes and parotid cells. International Journal of Molecular Medicine, 8, 37-42.
- Scruel, O., Vanhoutte, C., Sener, A. and Malaisse, W.J. (1998) Interference of D-mannoheptulose with D-glucose phosphorylation, metabolism and functional effects: Comparison between liver, parotid cells and pancreatic islets. Molecular and Cellular Biochemistry, 187, 113- 120. doi:10.1023/A:1006812300200
- Malaisse, W.J., Kadiata, M.M., Scruel, O. and Sener, A. (1998) Esterification of D-mannoheptulose confers to the heptose inhibitory action on D-glucose metabolism in parotid cells. Biochemistry and Molecular Biology International, 44, 625-633.
- Sener, A., Scruel, O., Louchami, K., Jijakli, H. and Malaisse, W.J. (1999) Inhibition of glucose-induced insulin release by 3-O-methyl-D-glucose: Enzymatic, metabolic and cationic determinants. Molecular and Cellular Biochemistry, 194, 133-145. doi:10.1023/A:1006955617718
- Courtois, P., Sener, A. and Malaisse, W.J. (2001) D-mannoheptulose phosphorylation by hexokinase isoenzymes. International Journal of Molecular Medicine, 7, 359-363.
- Sener, A., Giroix, M.-H. and Malaisse, W.J. (2002) Underestimation of D-glucose utilization as judged from the conversion of D-[3-3H]glucose to 3HOH. Diabetologia, 45, 1274-1280. doi:10.1007/s00125-002-0907-5
- Giroix, M.-H., Jijakli, H., Courtois, P., Zhang, Y., Sener, A. and Malaisse, W.J. (2006) Fructokinase activity in rat liver, ileum, parotid gland, pancreas, pancreatic islet, B and non-B islet cell homogenates. International Journal of Molecular Medicine, 17, 517-522.
- Scruel, O., Sener, A. and Malaisse, W.J. (1999) Hexose metabolism in pancreatic islets: Effect of D-glucose upon D-fructose metabolism. Molecular and Cellular Biochemistry, 197, 209-216. doi:10.1023/A:1006910201767
- Giroix, M.-H., Scruel, O., Ladrière, L., Sener, A., Portha, B. and Malaisse, W.J. (1999) Metabolic and secretory interactions between D-glucose and D-fructose in islets from GK rats. Endocrinology, 140, 5556-5565. doi:10.1210/en.140.12.5556
- Ladrière, L., Louchami, K., Laghmich, A., MalaisseLagae, F. and Malaisse, W.J. (2001) Labeling of pancreatic glycogen by D-[U-14C]glucose in hyperglycemic rats. Endocrine, 14, 383-397. doi:10.1385/ENDO:14:3:383
- Malaisse, W.J., Damhaut, P., Malaisse-Lagae, F., Ladrière, L., Olivares, E. and Goldman, S. (2000) Fate of 2- deoxy-2-[18F]fluoro-D-glucose in control and diabetic rats. International Journal of Molecular Medicine, 5, 525-532.
- Malaisse, W.J., Damhaut, P., Ladrière, L. and Goldman, S. (2000) Fate of 2-deoxy-2-[18F]fluoro-D-glucose in hyperglycemic rats. International Journal of Molecular Medicine, 6, 549-552.
- Sener, A., Jijakli, H., Zahedi Asl, S., Courtois, P., Yates, A.P., Meuris, S., Best, L.C. and Malaisse, W.J. (2007) Possible role of carbonic anhydrase in pancreatic islets: Enzymatic, secretory, metabolic, ionic, and electrical aspects. American Journal of Physiology, Endocrinology and Metabolism, 292, E1624-E1630. doi:10.1152/ajpendo.00631.2006
- Sener, A., Courtois, P., Meuris, S. and Malaisse, W.J. (2008) Carbonic anhydrase activity in parotid cells and pancreatic islets from Goto-Kakizaki rats. Metabolic and Functional Research on Diabetes, 1, 26-27.
- Youssef, R., Malaisse, W.J., Courtois, P. and Sener, A. (2003) Alteration of a-amylase secretion from rat parotid cells in the absence of extracellular bicarbonate. International Journal of Molecular Medicine, 12, 199-200.
- Delporte, C., Malaisse, W.J., Jurysta, C., Portois, L., Sener, A. and Carpentier, Y.A. (2007) Altered fatty acid pattern of phospholipids and triglycerides in the submandibular gland of w3-depleted rats. European Journal of Oral Sciences, 115, 103-110.
- Jurysta, C., Sener, A., Carpentier, Y.A. and Malaisse, W.J. (2006) Phospholipid and triglyceride content and fatty acid pattern in the submandibular gland of rats depleted in long-chain polyunsaturated w3 fatty acids. Acta Physicologica, 187, 2758-2761.
NOTES
*Corresponding author.

