Open Journal of Internal Medicine
Vol.2 No.2(2012), Article ID:19605,9 pages DOI:10.4236/ojim.2012.22010
Neisseria meningitis serogroup X outbreak in Burkina Faso, 2009-2010
![]()
1Centre Muraz, Bobo-Dioulasso, Burkina Faso
2Pôle Epidémiologie et Biostatistique, Institut de Recherche Experimentale et Clinique (IREC), Faculté de Santé Publique (FSP), Université Catholique de Louvain (UCL), Louvain, Belgique
3Agence de Médecine Préventive (AMP), Paris, France
4Institut de Recherche Santé et Société (IRSS), Faculté de Santé Publique (FSP), Université Catholique de Louvain (UCL), Louvain, Belgique
Email: *yaro_seydoy@yahoo.com, drabocisaly@yahoo.fr, meya_4@yahoo.fr, fati.kirakoya@uclouvain.be, jmueller@aamp.org, osanou@aamp.org, htall@aamp.org, pjaillard@aamp.org, blafourcade@aamp.org, Jean.Macq@uclouvain.be, annie.robert@uclouvain.be, jbouedraogo@gmail.com
Received 29 June 2011; revised 4 October 2011; accepted 18 October 2011
Keywords: Epidemiologic surveillance; pneumococcal; NmX Emergence; Meningitis; Burkina Faso
ABSTRACT
Background: Centre MURAZ of Bobo-Dioulasso (Burkina Faso) organized in 2009 and 2010 a system of Cerobro-Spinal Fluid (CSF) collection in eight pilot Districts as an initial step for the future Ministry of Health’s led strategy of individual surveillance in a context of meningococcal conjugate A vaccine introduction. Methods: CSF samples were analyzed with Polymerase Chain Reaction (PCR). This allowed for meningitis etiologies dynamics studies in the pilot Districts. Results: Because of geographical difficulties and lack of means, less than 40% of suspected cases had their CSF analyzed at PCR reference laboratory. In 2009, among confirmed cases at reference laboratory, Sp (Streptococcus pneumonia), NmA (Neisseria meningitis A) and Hib (Hemophilus influenzae b) were responsible respectively for 90%, 6.6% and 4.4% of cases. In 2010, serogroup distribution among confirmed cases was: Sp 62.7%, NmX 32.2% and NmA 5.1%. Sp which was continuously present in Burkina Faso takes more significant proportions, just as serogroup X which until there was sporadically encountered. The attack rates of NmX were tree to twelve times higher than for NmA in the two Districts where NmX has been notified. Conclusion: As a consequence of such results, efforts must be maintained in epidemiologic surveillance field and in reinforcement of laboratory capacities. Fast care should be guaranteed to patients with adequate antibiotics according to country national guideline and chemoprophylaxis measures should be undertaken among contacts of patients to prevent secondary cases. A plea must be made on one hand for pneumococcal vaccine introduction in Burkina Faso and on other hand towards manufacturers for taking into account serogroup X into meningococcal polyvalent vaccine composition. With this polyvalent vaccine including serougruop X, we suggested to conduct periodically mass campaign vaccination of people before the beginning of meningitis epidemiological season.
1. INTRODUCTION
African meningitis belt is characterized by severe meningitis epidemics due to meningococcal. This occurs in a context of high incidence of endemic cases during inter epidemic years. The majority of cases occur during dry season from January to April. In this area, until recently, majority of severe meningitis epidemics due to meningococcal were caused by serogroup A of Neisseria meningitis (NmA) [1-3]. Serogroup C was rarely involved in recent meningitis except in an epidemic reported in 1979 in Uper Volta, now Burkina Faso [3,4,6], that serogroup was rarely involved in current meningitis epidemics in meningitis belt countries [6,7]. In 2002, the first severe African epidemic of meningitis caused by Nm W135 occurred in Burkina Faso [8,9] and there was fear of rapid extension of this epidemic in the African meningitis belt. But eight years after, this extension didn’t occur and cases of meningitis W135 were relatively rarely identified in Burkina through national reinforced surveillance data system. Sporadic cases of meningitis caused by serogroupe X of Neisseria meningitis (NmX) were already reported in Niger in 1990 [10,11] and from 1995 to 2000 [12] and in Ghana in 2000 [13]. Since 2006, sporadic cases of NmX were also reported in some health Districts of Burkina Faso (Data from routine surveillance of Health Ministry not published). Burkina Faso was therefore logically engaged in meningitis fight caused by serogroup A. In December 2010, there was a national mass immunization campaign against this serogroup (NmA) with conjugate A vaccine. In Parallel, relatively significant proportions of notified case of meningitis during these two last years (2009-2010) were attributed to serogroup X (NmX). In addition, it is known that actually no vaccine against this serogroup exists. That could eventually constitute the beginning of acute bacterial meningitis epidemiologic trend change in Burkina Faso during the coming two to three years. The goal of this work is to show the significant increase in serogroup X meningitis case number in the epidemiologic trend of bacterial etiologies of meningitis in Burkina Faso, during 2010 epidemic season.
2. METHODS
2.1. Data Collection
Centre MURAZ set up a system of CSF collection in eight pilot Districts of Burkina Faso for contributing to prepare the field individual surveillance promoted by the Ministry of Health (MoH) after meningococcal conjugate A vaccine introduction. This program was set up by Centre MURAZ in collaboration with the Agence de Medé- cine Préventive (AMP) with a financial support of the French Ministry of Foreign Affairs (MAE) via Institut Pasteur of Paris through the Fonds de Solidarité Prioritaire (FSP). Throughout this system, some dispositions are taken so that all CSF carried out from suspected cases of meningitis on the eight pilot Districts level in the West part of the country can profit from PCR laboratory analysis. CSF conserved at ambient temperature during several days before are conveyed at Centre MURAZ PCR laboratory. Conditioning is done by triple packing system, in an isothermal container with ice boxes and the package is entrusted to conveyors of public transport. A communication network allows the information flow between District teams and laboratory team of Centre MURAZ. In parallel, national system of notification based on national guide of standard clinical meningitis case definition followed its course in the eight pilot Districts level. Biological confirmation of CSF is not necessary before case notification.
2.2. Laboratory Methods
In 2002, acute bacterial meningitis method diagnosis by PCR was transferred to Centre MURAZ. This diagnosis method by PCR comes in complement from traditional methods of diagnosis such as Gram, latex and Culture. These traditional methods are often not possible to realize at Districts level, on hand because of a lack of reagents in particular concerning latex, and on another hand because of the too long time before CSF arrival at the laboratory for culture. PCR method is used for the diagnosis of the three principal etiologies responsible of meningitis in Burkina: Neisseria meningitis, Streptococcus pneumoniae and Haemophilus influenza b according to well defined procedures’ [14,15]. An aliquot of each CSF sample or each Trans-Isolate medium supernatant is freeze-thawed, boiled for 5 min and centrifuged at 10,000 g for 10 min. One proceeds by a multiplex singletube PCR on 10 mL of the supernatant for amplification of the crgA genes of Nm [16], the lytA gene of S. pneumoniae [17] and the bexA gene of H. influenzae [18]. For genogrouping Nm positive specimens, a second PCR is performed for the amplification of the siaD genes for Nm serotypes B, C, Y and W135 and the open reading frame 2 of the gene mynB for NmA [16]. In 2004, a PCR protocol developed by the Neisseria Unit of Institut Pasteur of Paris for the detection of NmX was added to our range of genogrouping PCR for Nm serogroups A, B, C, Y, and W135. For NmX PCR Protocol, two primers were designed in the serogroup X capsule biosynthesis (xcbA) gene, one of the xcbABC clusters of three genes which are single for NmX and were confirmed like essential for the NmX capsule expression [19]. These 2 primers are primer X-10 5’-ACAGCCCATAAAACACCCGTATCATC-3’ and primer X-11 5’-GTGATTGGAATCTTGCAATATCGGT-3’, and they specifically amplify a 202— base pair DNA fragment. Any amplification was obtained from isolates of a collection of isolates from other serogroups [20].
3. RESULTS
The eight Districts notified at the national surveillance system level 656 of cumulative clinically diagnosed cases of meningitis from January to December 2009 and 695 cumulative cases from January to December 2010. The prevalence of clinical cases in each District is presented in figure 1. During the two years, Solenzo was the most concerned District, followed by Boromo and Sindou Districts. In 2009, CSF samples were performed for 256 (39.0%) of cumulative clinically diagnosed cases, and were analyzed at PCR laboratory of Centre MURAZ. Among theses, 91 cases (35.5%) revealed positive with an etiology of meningitis. Pneumococcus (Sp), Neisseria meningitidis A (NmA) and Hemophilus influenza b (Hib) accounted for 90%, 6.6% and 4.4% of positive cases respectively. Samples distribution according to District
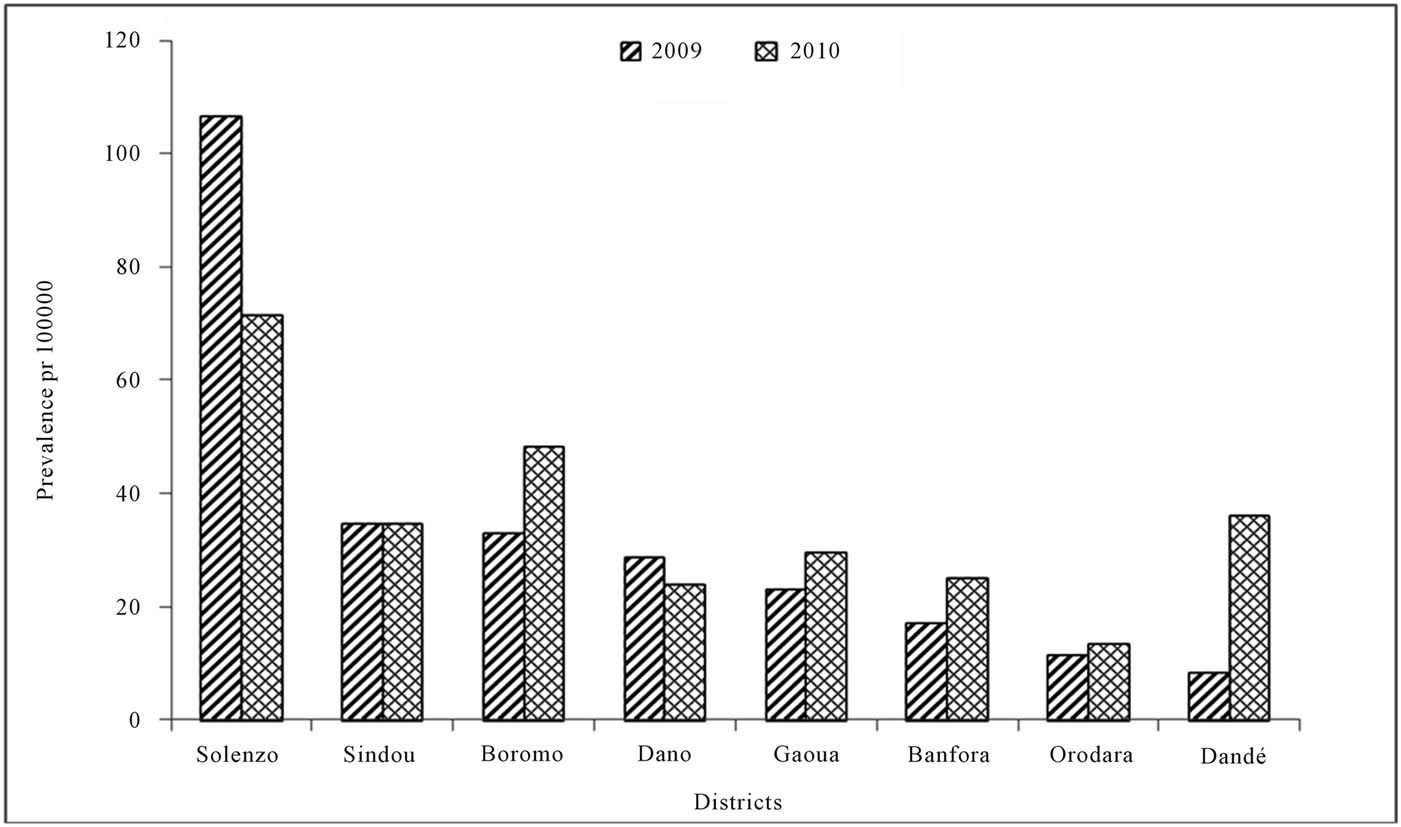
Figure 1. Prevalence of cumulative clinical cases in each Distict in 2009 and 2010.
source and identified serogroup is presented in table 1. In 2009, no NmX case had been identified at the eight pilot Districts level. The comparison between clinically diagnosed cases from national notification system level and cases which were analyzed by PCR according to District source in 2009 is presented in figure 2. The global attack rate of meningitis for the eight Districts was 32.8 for 100,000 inhabitants in 2009 (population size estimated at 1,970,692 inhabitants), with a global lethality of 15.1 per 100. The specific attack rate by serogroup was 4.1, 0.3, and 0.2 for 100,000 inhabitants for pneumococcus, Neisseria meningitidis A and Hemophilus influenza b respectively, when pooling over the eight Districts. Specific attack rate by serogroup and Districts are shown in figure 3. In 2010, from the 695 cumulative clinical diagnosed cases, 213 CSF samples (31.0%) have been sent to PCR laboratory of Centre MURAZ. Of these 213 CSF samples, 59 cases (27.7%) were positive to an etiology of meningitis. pneumococcus (Sp), Neisseria meningitidis serogroup X (NmX) and the Neisseria meningitidis sérogroupe A (NmA) represented, 62.7%, 32.2% and 5.1% of positive cases respectively. Solenzo District in Boucle du Mouhoun sanitary region has totalized (13 NmX cases) which represent twice more cases than Dandé District (6 NmX cases) in Hauts Bassins sanitary region. Samples distribution according to the District source and identified serogroup is represented in table 2. From January to December 2010, the global attack rate of meningitis for the eight Districts was 28.6 for 100,000 inhabitants (population size estimated at 1,988,015 inhabitants) with a global lethality of 20.8 per 100 over the same period. The specific attack rate by serogroup was 1.9; 0.9 and 0.2 for 100,000 inhabitants for the pneumococcus, Neisseria meningitidis X and Neisseria meningitidis A, respectively. Specific attack rates by identified serogroup and by Districts are shown in figure 4. In 2010, the comparison between notified cases on clinical symptom basis and cases which were analyzed by PCR by District are presented in figure 5.
4. DISCUSSIONS
Among suspected cases of meningitis notified at national surveillance system level by the eight Districts, less than 40% of CSF samples collected arrived at PCR laboratory of Centre MURAZ. This proportion is relatively weak owing to the fact that within this pilot project framework, some mechanisms had been set up to facilitate CSF collection and it’s routing on the eight Districts level. In 2006 epidemic season in Niger, on 4185 suspected cases notified by national surveillance system, the reference laboratory had received 2905 CSF Samples, which represented approximately 70% of suspected cases [20]. Within our study framework, CSF proportion from suspected cases of meningitis which profited from a PCR analysis is definitely better than the previous years where

Table 1. Distribution of serogroup within each district, January to December 2009.
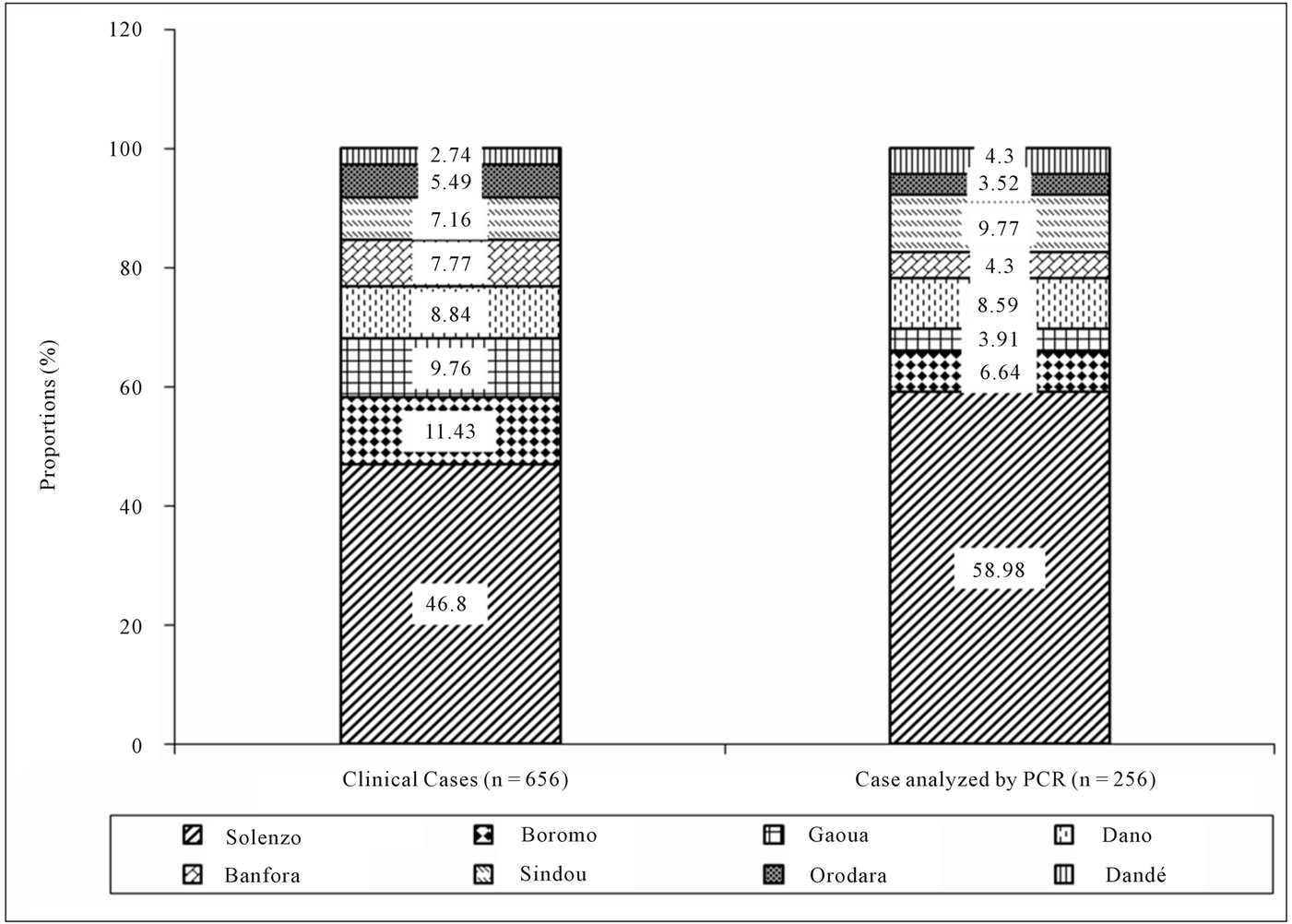
Figure 2. Distribution of notifed cases and case analyzed by PCR accross District, 2009.
the national directives recommended confirming the first ten cases to make it possible to take response measures. It remains however insufficient in the perspective of individual surveillance strategy after conjugate vaccine introduction whereby all CSF sample perform from any suspected case must necessary be analyze by a laboratory. It is possible to have difficulties to obtain CSF on the field from a suspected case by lumbar puncture, which is not an easy medical act. In this case, notification can be made without there being CSF sample to analyze by the

Figure 3. Attack rates by identified serogroup in each Districts, 2009.

Figure 4. Attack rates by identified serogroup in each Districts, 2010. *Dano, Gaoua, Orodara and Boromo didn’t send CSF for PCR in 2010.

Table 2. Distribution of serogroup within each District, January to December 2010.

Figure 5. Distribution of notifed cases and case analyzed by PCR accross District, 2010. *Dano, Gaoua, Orodara and Boromo didn’t sent CSF for PCR in 2010.
laboratory. However, there are obviously insufficiencies in cases notification and also difficulties on field level to ensure CSF collected routing towards reference laboratory. On reference laboratory level, it can be some difficulties per moment to ensure CSF analysis because of reagents ruptures in relation to disease extent. Serogroup X of Nesseria meningitis was described for the first time in years 1960 and was implied in sporadic cases of invasive meningitis through North America, Europe and Africa [21]. Meningococcal serogroup X was responsible for sporadic cases on some health Districts level of Burkina (Surveillance data not published). Since 2006, a report is made through surveillance system of relative fall of serogroup A case number of meningitis. On another hand, case number caused by pneumococcus which was always present in any season these last years [22], get important proportions, just as ascribable case number of serogroup X. Beside of isolated cases described more than ten years ago in Ghana (9 cases over a period of two years from 1998 to 2000) and in Niger (134 cases from 1995 to 2000), it is case number described during 2006 epidemic season in Niger which was most impressive where more half of confirmed cases of meningitis was due to serogroup X [20]. Niger is moreover a West African country which had described the greatest number of meningitis serogroup X cases, with the first cases isolated in 1982 [23]. Then after a silent of 8 years, 22 cases were described in 1990 [12,24]. Between 1995 and 2000, serogroup X meningitis cases were annually detected, with a peak of 83 cases in 1997 [12,25,26]. In 2005- 2006, serogroup X meningitis cases were confirmed in the West of Kenya [23], but before this period no case due to this serogroup X had been described in East Africa [27]. The cases described in Ghana between 1998 and 2000 coincided with the significant increase in carriage of serogroup X [13,21,26]. This increase in carriage and outbreak of serogroup X meningitis cases coincided with significant reduction in dominating serotype A in the epidemic plan. Later, a new serotype A took the top again, while the carriage and the number of serogroup X cases dropped [26].
In Africa, the outbreak of meningitis epidemics due to less frequent serogroup were often charged to mass campaigns immunization against the prevalent serogroup which is NmA. What would result in to facilitate outbreak and diffusion of others serogroups [21,28,29]. However, this hypothesis seems moderate, because in 2006, maximum of serogroup X meningitis cases occurred in a South western area of Niger who didn’t know an epidemic these last years and from which last immunization campaign goes back to before 2001 [20]. Molecular biology investigations have shown that NmX cases which have occurred in Kenya into 2006, was of sequence type ST5403 and differed from those described in Niger and Ghana which were sequences type ST181, ST5789 and ST751 [21,23,27]. NmX serotypes were described in Africa in the last forty years, during which they were responsible for sporadic cases particularly in Senegal in 1981, Niger in 1982, Chad in 1995 and Burkina Faso in 2003 [20]. Sequencing of cases described in 2010 in Burkina Faso is ongoing and will make it possible to see whether there is a bond with sequences which circulated in the Sub Saharan region or in East Africa. It is probable that evolution of serogroup X in Burkina Faso will be similar to that of W135. This serogroup emerged between 2000 and 2002 [8,30], then it remained limited to Burkina Faso, without epidemiological dissemination in the other neighboring countries, and knew a fast reduction in time in particular from 2003 [23,31]. Burkina Faso had known a similar situation in 1979 [4,6] with an emergence of serogroup C, geographically limited and which disappeared quickly in time. The proportion of serogroup X cases of meningitis noted in Burkina Faso into 2010 is a significant concern. Although this serogroup don’t have the same epidemic potential which is recognized to serogroup A until there [20,23], the reports observed, on one hand, during 2006 epidemic season in Niger [20] and on another hand, into 2010 in Burkina Faso, particularly in some health Districts such as Solenzo and Dandé make fear an epidemic risk of serogroup X explosion in African meningitis belt. In the image of what was made for the serogroup C and W135, potential epidemic of serogroup X took significant dimension to take into account within the conjugate A vaccine framework introduction in Burkina Faso, and in the other Sub Saharan countries [20,23,32]. A strong plea is necessary to be made so that vaccines manufacturers take into account this serogroup X in polyvalent vaccines composition. Otherwise, similar situations could be observed with the pneumococcal conjugate vaccine introduction, where serotypes not taken into account in vaccine composition replaced those eliminated by immunization [33]. In this context, it would be significant to reinforce surveillance in general and particularly the reinforcement of laboratories capacities to follow dynamoics etiologies responsible of meningitis after conjugate A vaccine introduction in Burkina Faso.
5. CONCLUSION
The significant increase of serogroup X case number in 2010 challenges the decision makers in regards of epidemic control strategies. The reinforcement of laboratories capacities is essential with complementarities between bacteriology and molecular biology, while the development of an epidemiologic prompt and powerful surveillance system based on all available means techniques of communication. Fast care should be guaranteed to patients with adequate antibiotics according to country national guideline and chemoprophylaxis measures should be undertaken among contacts of patients to prevent secondary cases. A strong plea will be made in favor of obtaining and introducing of meningococcal polyvalent vaccine including serogroup X. With this polyvalent vaccine including serougruop X, we suggested to conduct periodically mass campaign vaccinetion of people before the beginning of meningitis epidemiological seasons.
6. ACKNOWLEDGEMENTS
We thank responsible of Health Ministry of Burkina and the responseble of the height pilot Districts for their contribution for sending CSF samples at Centre MURAZ laboratory. Our thanks also go toward team of molecular biology laboratory of Centre MURAZ for the quality of analysis performed and toward the consortium study group of FSPmeningitis project. We thank also the Fonds de Solidarité Prioritaire (FSP) of the Ministry for Foreign Affairs of France (MAE) for the financial support to this program.
REFERENCES
- Lapeyssonnie, L. (1963) Cerebrospinal meningitis in Africa (in French). Bulletin of the World Health Organ, 28, 1-114.
- Greenwood, B. (1999) Manson lecture: Meningococcal meningitis in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene, 93, 341-353. doi:10.1016/S0035-9203(99)90106-2
- Molesworth, A.M., Thomson, M.C., Connor, S.J., Cresswell, M.P., et al. (2002) Where is the meningitis belt? Defining an area at risk of epidemic meningitis in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene, 96, 242-249. doi:10.1016/S0035-9203(02)90089-1
- World Health Organization (1998) Control of epidemic meningococcal disease. WHO practical guidelines. 2nd Edition, WHO, Geneva.
- Schuchat, A., Robinson, K., Wenger, J.D., Harrison, L.H., et al. (1997) Bacterial meningitis in the United States in 1995. The New England Journal of Medicine, 337, 970- 976. doi:10.1056/NEJM199710023371404
- Broome, C.V., Rugh, M.A., Yada, A.A., Giat, L., et al. (1983) Epidemic group C meningococcal meningitis in Upper Volta, 1979. Bulletin of the World Health Organ, 61, 325-330.
- Koumare, B., Bougoudogo, F., Cisse, M., Doumbia, T., et al. (1993) Bacteriological aspects of purulent meningitis in Bamako district: A propos of 1541 bacterial strains collected from 1979 to 1991 (in French). Bulletin de la Société de Pathologie Exotique, 86, 136-140.
- Parent du Chatelet, I., Traore, Y., Gessner, B.D., Antignac, A., et al. (2005) Bacterial meningitis in Burkina Faso: Surveillance using field-based polymerase chain reaction testing. Clinical Infectious Diseases, 40, 17-25. doi:10.1086/426436
- World Health Organization (2002) Meningococcal disease, serogroup W135, Burkina Faso: Preliminary report, 2002. Weekly Epidemiological Record, 77, 152-155.
- Campagne, G., Schuchat, A., Djibo, S., Ousseini, A., et al. (1999) Epidemiology of bacterial meningitis in Niamey, Niger, 1981-96. Bull World Health Organ, 77, 499-508.
- Etienne, J., Sperber, G., Adamou, A. and Picq, J.J. (1990) Epidemiological notes: Meningococcal meningitis of serogroup X in Niamey (Niger) (in French). Médecine Tropicale, 50, 227-229.
- Djibo, S., Nicolas, P., Alonso, J.M., Djibo, A., et al. (2003) Outbreaks of serogroup X meningococcal meningitis in Niger 1995-2000. Tropical Medicine & International Health, 8, 1118-1123. doi:10.1046/j.1360-2276.2003.01126.x
- Gagneux, S.P., Hodgson, A., Smith, T.A., Wirth, T., et al. (2002) Prospective study of a serogroup X Neisseria meningitidis outbreak in Northern Ghana. The Journal of Infectious Diseases, 185, 618-626. doi:10.1086/339010
- Sidikou, F., Djibo, S., Taha, M.K., Alonso, J.M., et al. (2003) Polymerase chain reaction assay and bacterial meningitis surveillance in remote areas, Niger. Emerging Infectious Diseases, 9, 1486-1488.
- Chanteau, S., Sidikou, F., Djibo, S., Moussa, A., et al. (2006) Scaling up of PCR-based surveillance of bacterial meningitis in the African meningitis belt: Indisputable benefits of multiplex PCR assay in Niger. Transactions of the Royal Society of Tropical Medicine and Hygiene, 100, 677-680. doi:10.1016/j.trstmh.2005.09.006
- Taha, M.K. (2000) Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. Journal of Clinical Microbiology, 38, 855-857.
- Garcia, P., Garcia, J.L., Garcia, E. and Lopez, R. (1986) Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene, 43, 265-272. doi:10.1016/0378-1119(86)90215-5
- Falla, T.J., Crook, D.W., Brophy, L.N., Maskell, D., et al. (1994) PCR for capsular typing of Haemophilus influenzae. Journal of Clinical Microbiology, 32, 2382-2386.
- Tzeng, Y.L., Noble, C. and Stephens, D.S. (2003) Genetic basis for biosynthesis of the (alpha 1→4)-linked N-acetyl-d-glucosamine 1-phosphate capsule of Neisseria meningitidis serogroup X. Infection and Immunity, 71, 6712-6720. doi:10.1128/IAI.71.12.6712-6720.2003
- Boisier, P., Nicolas, P., Djibo, S., Taha, M.K., et al. (2007) Meningococcal meningitis: Unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clinical Infectious Diseases, 44, 657-663. doi:10.1086/511646
- Gagneux, S., Wirth, T., Hodgson, A., Ehrhard, I., et al. (2002) Clonal groupings in serogroup X Neisseria meningitidis. Emerging Infectious Diseases, 8, 462-466.
- Gesner, B.D., Mueller, J.E. and Yaro, S. (2010) African meningitis belt pneumococcal disease epidemiology indicates a need for an effective serotype 1 containing vaccine, including for older children and adults. BMC Infectious Diseases, 10, 10-22. doi:10.1186/1471-2334-10-22
- David, M.M., Guillermo, P., Judith, M., Charles, N., et al. (2009) Epidemiology and risk factors for serogroup X Meningococcal Meningitis during an outbreak in Western Kenya, 2005-2006. The American Journal of Tropical Medicine and Hygiene, 80, 619-624.
- Etienne, J., Sperber, G., Adamou, A. and Picq, J.J. (1990) Epidemiologic notes: Meningococcal meningitis of serogroup X in Niamey (Niger). Médecine Tropicale, 50, 227-229.
- Campagne, G., Schuchat, A., Djibo, S., Ousséini, A., et al. (1999) Epidemiology of bacterial meningitis in Niamey, Niger, 1981-96. Bulletin of the World Health Organ, 77, 499-508.
- Leimkugel, J., Hodgson, A., Forger, A.A., et al. (2007) Clonal waves of Neisseria colonization and disease in the African meningitis belt: Eight year longitudinal study in Northern Ghana. PLoS Medicine, 4, 0535-0544.
- Materu, S., Cox, H.S., Isaakidis, P., Baruani, B., et al. (2007) Serogroup X in meningococcal disease, Western Kenya. Emerging Infectious Diseases, 13, 944-945. doi:10.3201/eid1306.070042
- Taha, M.K., Deghmane, A.E., Antignac, A., Zarantonelli, M.L., Larribe, M. and Alonso, J.M. (2002) The duality of virulence and transmissibility in Neisseria meningitidis. Trends Microbiology, 10, 376-382. doi:10.1016/S0966-842X(02)02402-2
- MacLennan, J.M., Urwin, R., Obaro, S., Griffiths, D., et al. (2000) Carriage of serogroup W-135, ET-37 meningococci in the Gambia: Implications for immunisation policy? The Lancet, 356, 1078. doi:10.1016/S0140-6736(00)02734-3
- Lingappa, J.R., Al-Rabeah, A.M., Hajjeh, R., Mustafa, T., et al. (2003) Serogroup W135 meningococcal disease during the Hajj, 2000. Emerging Infectious Diseases, 9, 665-671.
- Traoré, Y., Njanpop-Lafourcade, B.M., Adjogble, K.L., Lourd, M., et al. (2006) The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002-2005. Clinical Infectious Diseases, 43, 817-822.
- WHO (2007) Improved meningitis vaccine for Africa could signal eventual end to deadly scourge. Weekly Epidemiological Record, 24, 222-224.
- Singleton, R.J., Hennessy, T.W., Bulkow, L.R., Hammitt, L.L., et al. (2007) Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. The Journal of the American Medical Association, 297, 1784-1792. doi:10.1001/jama.297.16.1784
NOTES
*Corresponding author.

