Open Journal of Inorganic Chemistry
Vol.2 No.2(2012), Article ID:18646,9 pages DOI:10.4236/ojic.2012.22003
Synthesis and characterization of a novel schiff base metal complexes and their application in determination of iron in different types of natural water
![]()
1Chemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt
2Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt
Email: *khalil62@yahoo.com
Received 23 October 2011; revised 20 January 2012; accepted 2 February 2012
Keywords: Novel Schiff base; Transition Metal Complexes and Natural Water
ABSTRACT
A novel and simple approach to the synthesis of macrocyclic Schiff base ligand resulted from the condensation of bisaldehyde and ethylenediamine was prepared (7, 8, 15, 16, 17, 18-hexahydrodibenzo (a, g) (14) annulene) (L) and its complexes were synthesized and characterized using different physicochemical studies as elemental analysis, FT-IR, 1H NMR, conductivity, magnetic properties, thermal analysis, and their biological activities. The spectroscopic data of the complexes suggest their 1:1 complexe structures which are investigated by elemental analysis, FT-IR, 1H NMR, conductivity, magnetic properties, thermal analysis, and their biological activities. The spectroscopic studies suggested the octahedral structure for the all complexes. The spectroscopic data of the complexes suggest their structure in which (N2O2) group act as a tetradentate ligand and two chlorides as monodentate ligands. Also electronic spectra and magnetic susceptibility measurements indicate octahedral structure of these complexes. The synthesized Schiff base and its metal complexes also were screened for their antibacterial and antifungal activity. Here we report the effect of a neutral chelating ligand on the complexation with iron to determine it in different types of natural water using recovery test. The activity data show that the metal complexes to be more potent/ antibacterial than the parent Schiff base ligand against one or more bacterial species.
1. INTRODUCTION
A large number of Schiff bases and their complexes have been investigated for their interesting and important properties, such as their ability to reversibly bind oxygen [1], catalytic activity in the hydrogenation of olefines [2], photochromic properties [3,4] and complexing ability towards some toxic metals [5]. Schiff bases are a special class of ligands with a variety of donor atoms exhibiting interesting coordination modes towards various metals [6- 8]. The azomethine linkage in Schiff bases is responsible for the biological activities such as antitumor, antibacterial, antifungal and herbicidal activities [9]. Designing a suitable polydentate Schiff base ligand to combine with a metal ion along with pseudohalide anion has opened a new area of synthesizing metal complexes of particular choice [10]. Such complexes are readily assembled from diamines and various salicylaldehyde derivatives and are amenable to combinatorial syntheses [11]. Metal Schiff base complexes have been well known for their easy synthesis, stability and wide application [12-14]. A large number of Schiff base (N2O2) complexes have been reported so far, and their catalytic and biological properties have been studied intensively [15-21]. Numerous techniques were available in iron determination have been reported [22-24]. However, these techniques were tedious need time and rigid conditions. Also due to the very low concentrations of iron, lead and chromium in natural water samples, their determinations demand very sensitive analytical techniques including ET-AAS, ICP-MS, ICPAES and XRF. However, relative to flame AAS, these techniques have some disadvantages such as its high cost, slowness and greater proneness to matrix interferences as well as their high sensitive advantages [25]. The present study was under taken to throw more light on the chelation behavior of Schiff base (L) towards some transition elements, which may help in more understanding of the mode of chelation of the new Schiff base towards metals and tried to used in the recovery test with a purity of more than higher than 96% for the metal ion.
2. EXPERIMENTAL
2.1. Materials and Solutions
The chemicals used included metal (II) and (III) chlorides. Solvents of absolute ethyl alcohol, diethylether, dimethylformamide and ethylenediamine were spectroscopic pure and purchased from BDH. A 10–4 M solution of the metal salts (CrCl3.6H2O (0.341 g/L), CoCl2.6H2O (0.119 g/L), NiCl2.6H2O (0.119 g/L) (BDH), FeCl3.6H2O (0.117 g/L) (Prolabo), CuCl2.2H2O (0.085 g/L) (Sigma) and ZnCl2.2H2O (0.086 g/L) (Ubichem)) were prepared by dissolving the accurately weighed amounts of the metal salts in the appropriate volume of de-ionized water. The metal salt solutions were standardized by the recommended procedures [26].
2.2. Instrumentation
Elemental microanalyses of the separated solid chelates for C, H, N and Cl were performed at the Microanalytical center, Cairo University. The molar conductance of the complexes in DMF was measured using Sybron-Barnstead conductometer (Meter-PM.6, E = 3406). Infrared spectra were recorded on a Perkin-Elmer FT-IR type 1650 spectrophotometer. The spectra were recorded as KBr pellets. The UV-vis absorption spectra were measured on a Shimadzu 3101pc spectrophotometer. The molar magnetic susceptibility was measured on powdered samples using the Faraday method. The diamagnetic corrections were made by Pascal’s constant and Hg[Co(SCN)4] was used as a calibrant. The mass spectra were recorded by the EI technique at 70 eV using MS- 5988 GS-MS Hewlett-Packard instrument. The H1NMRspectra were recorded using 300 MHz Varian-Oxford Mercury. The thermal analyses were carried out in dynamic nitrogen atmosphere (20 mL·min–1) with a heating rate of 10˚C·min–1 using Shimadzu TG-60H thermal analyzer. XPD analyses were carried out by using Philips Analytical X-Ray BV, diffractometer type PW 1840. Radiation was provided by copper target (Cu anode 2000 W) high intensity X-ray tube operated at 40 KV and 25 mA. All spectrophotometric measurements were preformed using a Unicam UV-2 spectrophotometer using 10 mm quartz cell and a blank solution as a reference. The pH measurements were made using HANNA pH meter.
2.3. Synthesis of the Compounds
2.3.1. Synthesis of Bisaldehyde
Salicylaldehyde (20 mmol) was dissolved in hot ethanolic KOH (prepared by dissolving 1.12 g (20 mmol) of KOH in 20 ml of absolute ethanol), and the solvent was then removed in vacuo. The remaining material was dissolved in DMF (15 ml) and the appropriate 1,2-dibromoethane (10 mmol) was added. The reaction mixture was refluxed for 5 min during which KCl was separated. The solvent was then removed in vacuo and the remaining materials was washed with water and purified by crystallization.
2.3.2. Synthesis of Schiff Base
Hot solution (60˚C) of ethylenediamine (0.399 g, 6.67 mmol) in 50 mL ethanol/DMF mixture as 1:1 mole ratio mixed with hot solution (60˚C) of bisaldehyde (1.6 g, 6.67 mmol) in the same solvent and the reaction mixture was left under heating for 30 min. A solid mass separated was collected and washed with diethylether. Crystallization was done with ethanol and then dried over CaCl2. The pale yellow ligand; L, (yield, 82%) was collected.
2.3.3. Synthesis of Metal Complexes
All the new complexes were prepared by adding hot ethanolic/DMF solution in a 1:1 molar ratio (60˚C) of metal (II)/(III) (1 mmol) to hot solution (60˚C) of ligand in the same solvent. The solution was stirred with heating for one hour whereupon the complexes precipitated then filtered and left for drying. A solid residue was separated and washed by diethylether. Crystallization was done with methanol and the complexes dried over anhydrous CaCl2 and the metal contents were determined compleximetrically.
2.4. Biological Activity
The standard disc-agar diffusion method [27] was followed to determine the antibacterial and antifungal activeity of the synthesized compounds. The tested compounds were dissolved in DMF (which have no inhibittion activity), to get concentrations of 100 mg/mL. Uniform size filter paper disks (3 disks per compound) were impregnated by equal volume (0.1 mL) from the specific concentration of dissolved tested compounds and carefully placed on incubated agar surface. After incubation for 48 h at 37˚C, inhibition of the organisms which evidenced by clear zone surround each disk was measured and used to calculate mean of inhibition zones.
2.5. The Recovery Procedure
Water samples were collected according to the recommended standard methods [28]. Water samples were spiked with a definite concentration of Fe(III) soluation and 1 ml of 10–4 M of the Schiff base was added. After adjusting the pH with HNO3 and/or NaOH, the solution was shaked well and transferred to the measurement cell to ensure complete complexation. The concentration of metal ion determined spectrophotometrically and obtained the recovery percentage.
3. RESULTS AND DISCUSSION
The results of elemental analyses of the ligand and its complexes are in good agreement with those required by the proposed formulae giving in Table 1. The molecular modeling of Schiff base ligand (using Chem3D ultra 8.0 program) shows that the bond lengths of all bonds in the left and right hand sides are typical due to the similarity of moieties on the two sides. Mass spectrum (Figure 1) of the new Schiff base showed a signal with m/z = 301 which is close to the calculated formula m/z = 294, supporting the structures of the Schiff base. The fragments observed were in good agreement with the proposed formula (Scheme 1).
3.1. Molar Conductance Measurements
The metal (II)/(III) complexes were dissolved in DMSO and the molar conductivities of 10–3 M of their solutions were measured at room temperature. The conductance values of the M(III) complexes support their electrolytic nature (1:1) types while M(II) complexes are non ionic in nature and non electrolytes. The data are listed in Table 1.
3.2. IR Spectral Studies
IR spectra in the region 4000 - 400 cm–1 have been recorded for the ligand and its complexes and the assignments of the observed frequencies have been made to specific group vibrations by comparison with the spectra of related complexes. The IR spectrum of the ligand showed a shoulder at 1597 cm–1 assigned to υ(C = N) of the azomethine stretching vibration, beside a band at 1049 cm–1 due to C-O-C stretching frequency. These two bands are shifted to higher or lower wave numbers in the complexes indicating the participation of the azomethine nitrogen in coordination [29] and the oxygen of the ether group, Table 2. On the other hand, the IR spectra of the complexes exhibited new non-ligand bands in the range 432 - 467 cm–1 and in the range 492 - 594 cm–1 assigned as M-O and M-N stretching vibrations, respectively. Therefore, it can be concluded that (L) ligand binds to the metal ions through azomethine N and the ether O and the ligand behaves as neutral tetradentate ligand.

Table 1. Analytical and physical data of L ligand and its metal complexes.
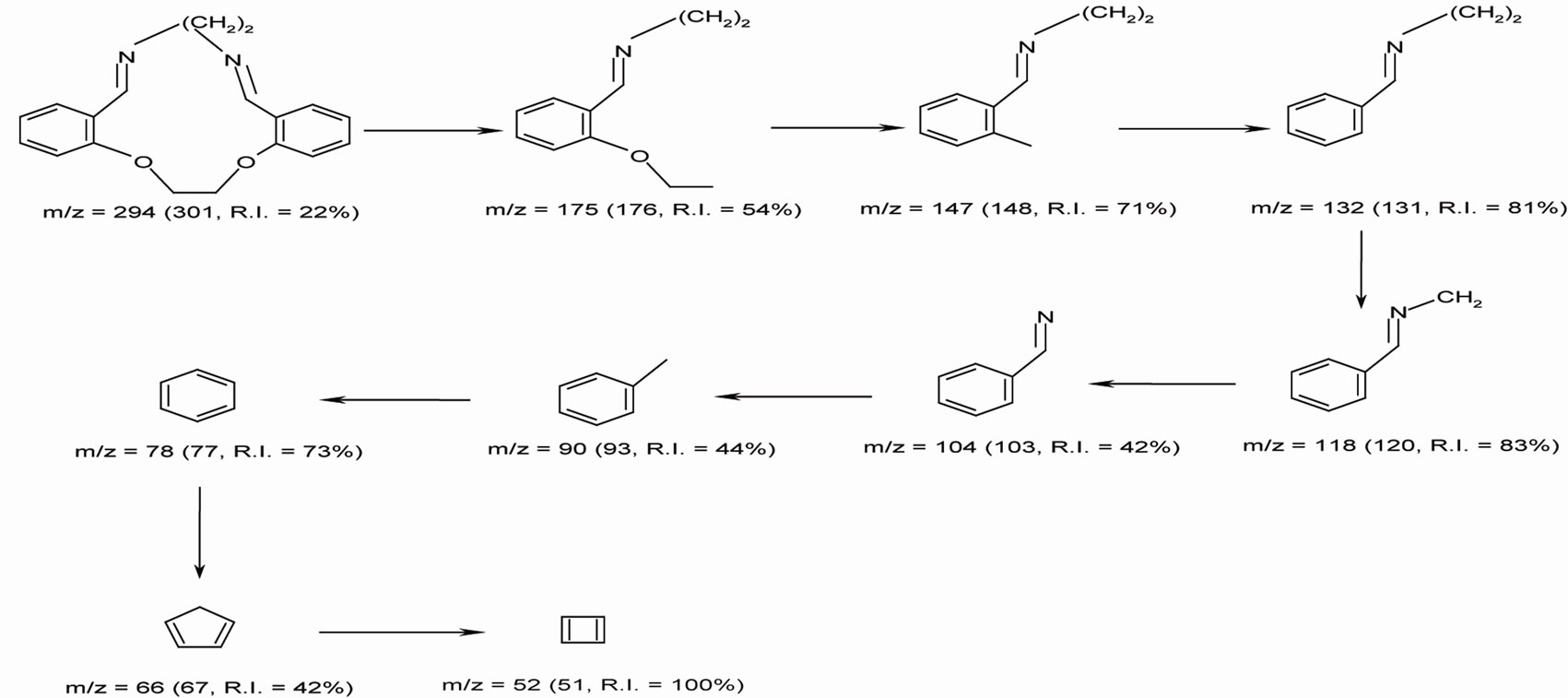
Scheme 1. Mass fragmentation pattern of L ligand.
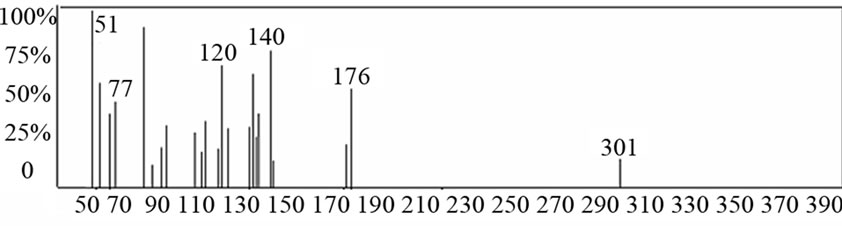
Figure 1. Mass spectra of Schiff base ligand (L).
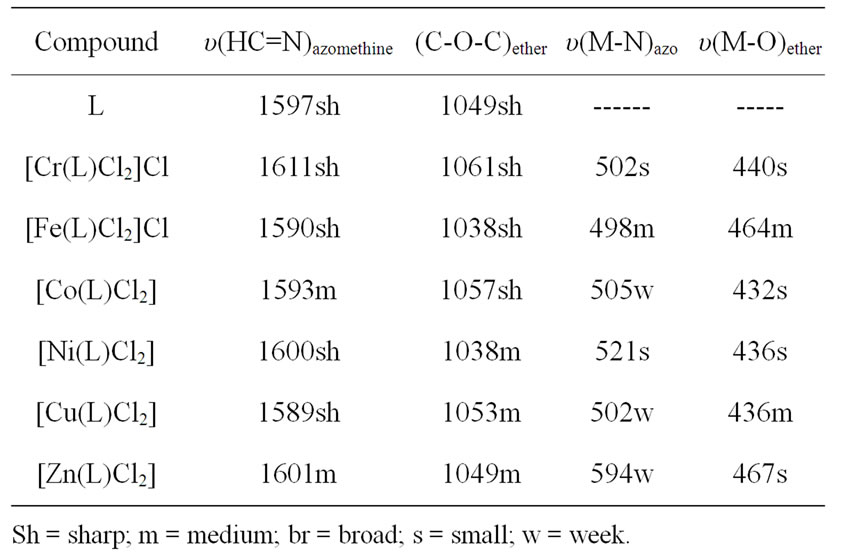
Table 2. IR data (4000 - 400 cm–1) of L ligand and its metal complexes.
3.3. H1NMR Spectra
The H1NMR spectra of Schiff base ligand (L) is recorded in d6-dimethylsulfoxide (DMSO) solution using tetramethylsilane (TMS) as internal standard. The chemical shifts of the different types of protons found in the H1NMRspectra of the Schiff base ligand is compared with its diamagnetic Zn(II) complex and shown in Table 3. It is found that while the H1NMRsignal of (HC=N) is shifted due to complexation, the signals due to the ether CH2-O does not show a significant shift in the chelation mode.
3.4. Electronic Absorption Spectra and Magnetic Moments of the Metal Complexes
The Uv-vis spectrum of the ligand showed two bands at 330 and 370 nm which are assigned to π-π* and n-π*, transition, respectively. The Uv-vis spectra of 10–4 M of the metal complexes, Figure 2, display similar absorption spectra of the ligand which are shifted to lower and higher wavelengths beside a decrease or disappearance of the peak dye to n-π* transition which confirm the coordination through azomethine nitrogen. On the other hand, π-π* transition showed shoulder at 337 nm with new band at 320 nm due to complexation. Also the d-d transition in this type of complexes may appear above 500 nm but does not appear due to the low intensity of the d-d transition. The data listed in Table 1 summarized the measured magnetic moment values. These data support the octahedral geometry of the complexes.

Table 3. H1NMRspectral data of the organic ligand and its metal chelates.

Figure 2. Uv-vis spectrum of the Schiff base and its metal complexes.
3.5. Thermal Analyses (TG and DTG) Studies
The Schiff base ligand exhibits a first estimated mass loss of 13.33% (calcd. 12.93%) at 25˚C - 140˚C, which may be attributed to the liberation of 2NH3 and 2H2 molecules as gases. In the following stages within the temperature range from 140˚C - 750˚C, the organic part C17H8 with CO2 gas are lost with an estimated mass loss of 86.67% (calcd. 87.07%). The thermogram of Fe(III)-L chelate show three decomposition steps within the temperature range from 50˚C - 790˚C. The first step of decomposition within the temperature range from 50˚C - 245˚C corresponds to the loss of 2HCl gases with an estimated mass loss of 16.18% (calcd. 16.00%). While the second step occurs within the temperature range from 245˚C - 520˚C and corresponds to the removal of HClCO and 2NH3 as gases with a mass loss of 21.91% (calcd. 21.58%). The final step (520˚C - 790˚C) corresponds to the removal of the organic part of the ligand leaving metal oxide as a residue. The overall weight loss amounts to 84.37% (calcd. 84.27%). The TG curve of the cobalt complex shows five stages of decomposition within the temperature range of 25˚C - 705˚C. The first three stages at 25˚C - 270˚C correspond to the loss of 2HCl as gases in the first step and NO and NH3 as gases in the next steps, while the final stages involve the loss of organic molecule leaving the metal oxide as residue. The overall weight loss amounts to 82.90% (calcd. 82.33%). The Cu(II) complex decomposed in four steps. The overall weight loss amounts to 82.22% (calcd. 81.45%), Table 4 and Figure 3.

Table 4. Thermoanalytical results of Schiff base and its metal complexes.
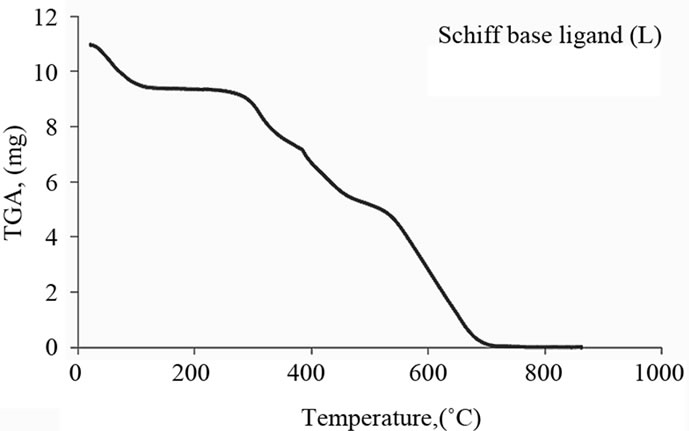 (a)
(a) (b)
(b) (c)
(c)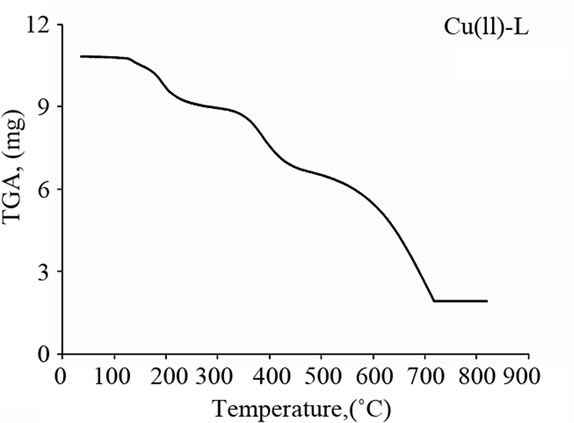 (d)
(d)
Figure 3. Thermal analyses of (a) Schiff base ligand (L), (b) Fe(III)-L, (c) Co(II)-L and (d) Cu(II)-L. complexes.
3.6. Calculation of Activation Thermodynamic Parameters
The thermodynamic activation parameters of decomposition processes of the complexes are evaluated graphically by employing the Coats-Redfern relation [30].
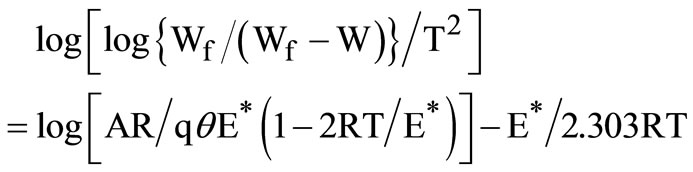
where Wf is the mass loss at the completion of the reaction, W is the mass loss up to temperature T; R is the gas constant, E* is the activation energy in KJ/mol and q is the heating rate. Since, l-2RT/E* @ l, a plot of the left hand side of the equation against 1/T was drawn and the data obtained are given in Table 5. The high values of the activation energies reflect the thermal stability of the complexes. The entropy of activation is found to have negative values in all the complexes which indicate that the decomposition reactions proceed with a lower rate than the normal ones.
3.7. X-Ray Diffraction
The trend of the curves decreases from maximum to minimum intensity indicating that all the metal complexes are amorphous in nature in the present metal-ligand formation except the Cr(III), Ni(II), Cu(II) and Zn(II) chelates they have crystalline phase.
3.8. Structures Interpretation
On the basis of the above observations, octahedral geometry is suggested for the investigated complexes. The structures of the complexes are shown in Figure 4.
Table 5. Thermodynamic data of the thermal decomposition of the Schiff base ligand and its metal complexes.
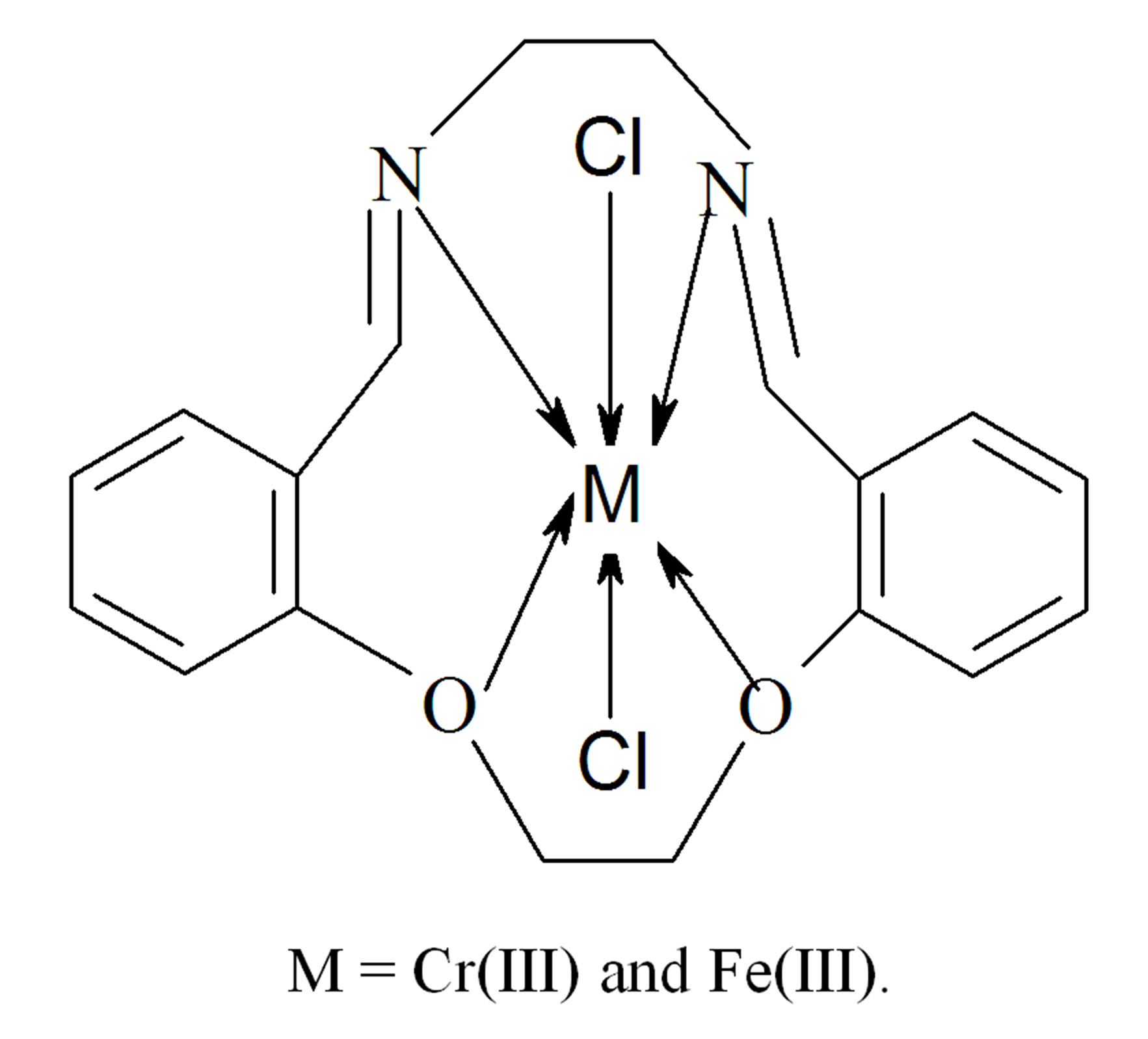
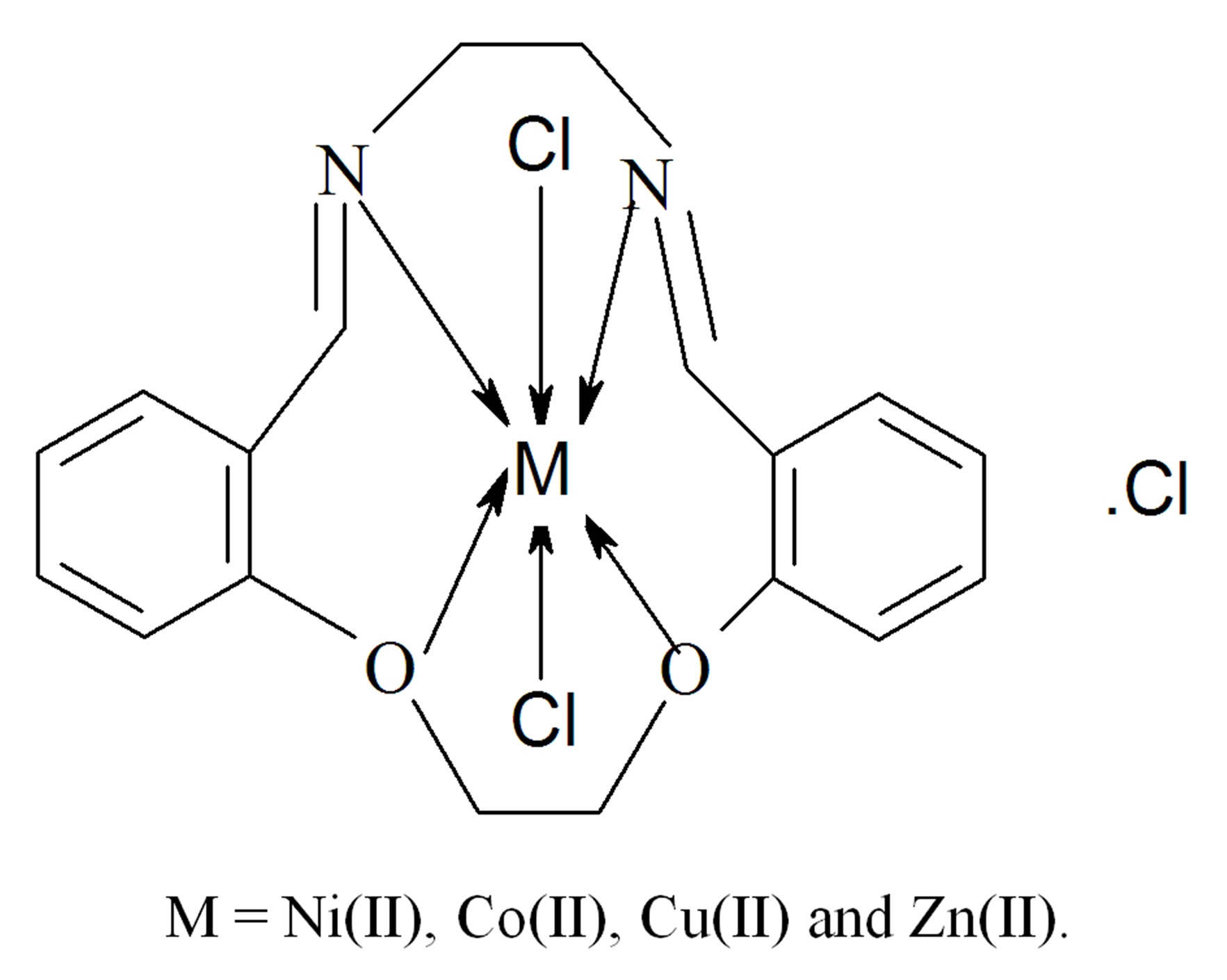
Figure 4. Structural formula of the metal complexes.
3.9. Biological Activity
The main aim of the production and synthesis of any antimicrobial compound is to inhibit the causal microbe without any side effects on the patients. In addition, it is worthy to stress here on the basic idea of applying any chemotherapeutic agent which depends essentially on the specific control of only one biological function and not multiple ones. The antibacterial activity of the parent Schiff base and its metal complexes against Aspergillus flavus and Canidia albicans fungi and, Staphylococcus (G+) and E. coli (G−) bacteria was tested in order to asses their potential antimicrobial agents. The biological activeity of the Schiff base ligand and its metal complexes were also compared with tetracycline (Antibacterial agent) and Amphotericin B (Antifungal agent). The data are listed in Table 6 and Figure 5. According to the data it can be seen that the ligand did not show any biological activity, while all the complexes showed activity against Staphylococcus (G+) and E. coli (G−) bacteria and Canidia albicans fungi. On the other hand, no activity is observed for Aspergillus flavus fungi by the ligand or the complexes [31]. This means that the activity of the newly prepared Schiff base against different microorganisms is enhanced with chelation with different biological active metals.
4. APPLICATION
A New Spectrophotometric Determination to Fe(III) Ions in Natural Water
The UV-vis spectrum of the Fe(III) solution in the presence of the Schiff base ligand was measured from 250 to 900 nm at an interval of 1 nm and an average measuring time of 1 s per wavelength and the maximum wavelength is found at 313 nm. Several factors influence on the recovery method are studied for [Fe(III)-L] system in order to achieve maximum recovery. The optimum factors are pH = 3, 25˚C, 1:1 (M:L) metal to ligand ratio and 30 min. In order to assess the applicability of the proposed method to recover Fe(III) from spiked water samples, the effect of some foreign ions was also investigated. These foreign ions were selected on the basis that they are normally present in natural water samples. Solutions containing various amounts of foreign ions, Fe(III), and the ligand were subjected to the described spectrophotometric procedure. The tolerable amounts of each ion, given a maximum error up to 1.23% in the recovery, are summarized in Table 7. As can be seen, all the investigated foreign ions with a relatively high concentration have no adverse effect on the ferric ions determinate.
Recovery tests were carried out on different types of water samples in order to assess its accuracy. The selection of these samples was done in a way to provide a wide variety of sample matrices characterized by different types of interferents. Solutions of water samples containing Fe(III) with varying a concentration were recovered with the newly Schiff base synthesized under the recommended conditions and the data listed in Table 8 showed satisfactory recovery of Fe(III). The standard deviation and relative standard deviation of the method were also calculated and their values indicate that, this method has a greater accuracy where the small values for the standard deviation attest to the procedure’s fairly high degree of precision. Therefore, the synthesis of these complexes might be established a new line for search to new ligand for determination of ferric ion in natural water.

Table 6. Biological activity of L and its metal complexes.
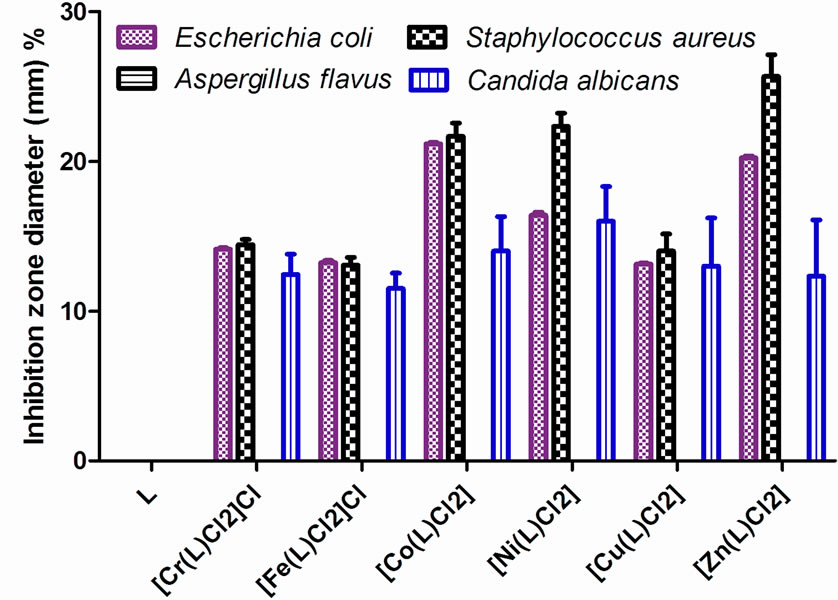
Figure 5. Percent of inhibition zone diameter (mm) relative to used standard vs. the Schiff base ligand (L) and its metal complexes.
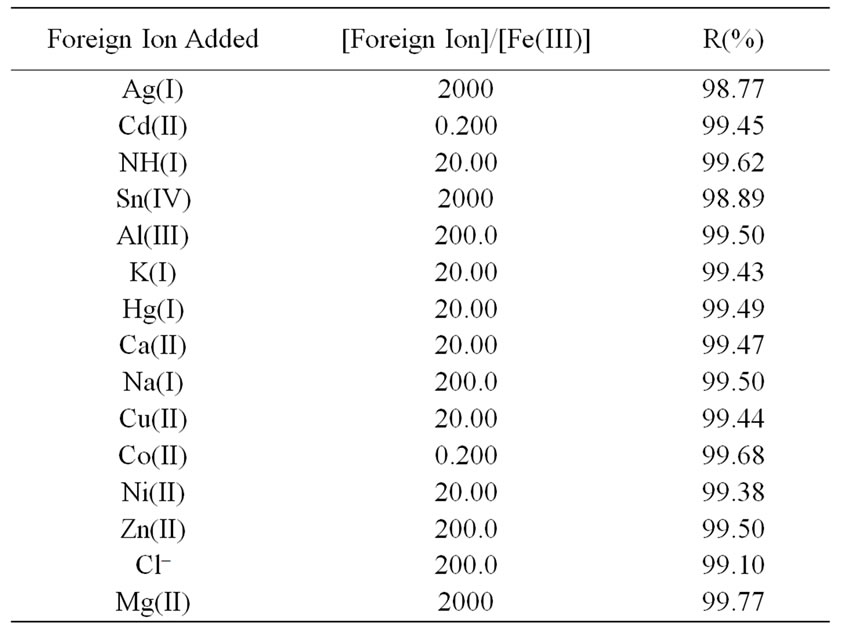
Table 7. Effect of some foreign ions on the recovery of 5 × 10–4 mol·L–1 Fe(III) using 5 × 10–4 mol·L–1 of L ligand at pH = 3, t = 30 min, T = 25˚C and λmax = 313 nm.
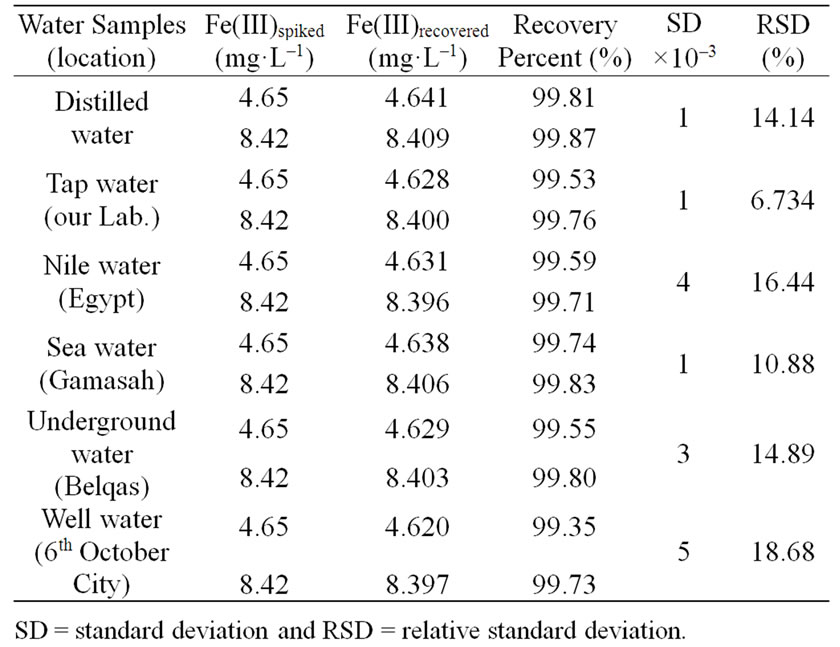
Table 8. Recovery percent (%) of Fe(III) in spiked natural water samples using 5 × 10–4 mol·L–1 at pH = 3, t = 30 min, T = 25˚C and λmax = 313 nm.
5. CONCLUSION
Synthesis and characterization of complexes containing N2O2-donor tetradentate Schiff base ligand have been described in this paper. The spectroscopic data of the complexes give good evidence of proposed structure. The results of the antibacterial screening of the test compounds indicate mild to moderate bactericidal activities and Canidia albicans fungi. Spectrometry procedure used to determine of iron (III) in different types of natural water within recovery test including with stable solution of the newly synthetic Schiff base as selective reagent by highly sensitive, selective and simple method than others.
REFERENCES
- Chen, D. and Martell, A.E. (1987) Dioxygen affinities of synthetic cobalt Schiff base complexes. Inorganic Chemistry, 26, 1026-1987. doi:10.1021/ic00254a013
- Collman, J. and Hegedus, L.S. (1980) principles and application of organotransition metal chemistry. University Science Book, Sausalito.
- Zhao, J., Zhao, B., Liu, J., Xu, W. and Wang, Z. (2001) Spectroscopy study on the photochromism of Schiff bases N,N’-bis(salicylidene)-1,2-diaminoethane and N,N’-bis- (Salicylidene)-1,6-exanediamine. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 57, 149- 154. doi:10.1016/S1386-1425(00)00353-X
- Zgierski, M.Z. and Grabowska, A. (2000) Theoretical approach to photochromism of aromatic Schiff bases: A minimal chromophore salicylidene methylamine. Journal of Chemical Physics, 113, 7845. doi:10.1063/1.1316038
- Sawodny, W.J. and Riederer, M. (1977) addition compounds with polymeric chromium(ii)-schiff base complexes. Agnewandate Chemie, 16, 859-860. doi:10.1002/anie.197708591
- Panda, B.K. and Chakravorty, A. (2005) spectroscopic properties of inorganic and organometallic compounds. Journal of Organometallic Chemistry, 690, 3169-3175. doi:10.1016/j.jorganchem.2005.04.012
- Krishnapriya, K.R. and Kandaswamy, M. (2005) Coordination properties of a dicompartmental ligand with tetraand hexadentate coordination sites towards copper(II) and nickel(II) ions Polyhedron. Polyhedron, 24, 113-120. doi:10.1016/j.poly.2004.10.010
- Kumar, K.N. and Ramesh, R. (1999) Synthesis of lanthanide(III) complexes of chloroand bromo-substituted 18-memberedtetraazamacrocycles. Polyhedron, 18, 1561- 1568.
- Rekha, S. and Nagasundara, K.R. (2006) Complexes of the Schiff base derived from 4-amino-phenyl benzimidazole and 2,2’-dehydropyrollidene-N-aldehyde with Zn(II), Cd(II) and Hg(II) halides. Indian Journal of Chemistry, 45, 2421-2425.
- Cotton, F.A., Wilkinson, G., Murillo, C.A. and Bochmann, M. (1999) Advanced inorganic chemistry. 6th edition, Wiley, New York.
- Shearer, J.M. and Rokita, S.E. (1999) Diamine preparation for synthesis of a water soluble Ni(II) salen complex. Bioorganic & Medicinal Chemistry Letters, 9, 501-504. doi:10.1016/S0960-894X(99)00020-7
- Singh, K., Barwa, M. S. and Tyagi, P. (2006) Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. European Journal of Medicinal Chemistry, 41, 147-153. doi:10.1016/j.ejmech.2005.06.006
- Majumder, A., Rosair, G.M., Mallick, A., Chattopadhyay, N. and Mitra, S. (2006) Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N,N,O-tridentate Schiff base N-2-pyridylmethylidene-2- hydroxy-phenylamine. Polyhedron, 25, 1753-1762.
- Freiria, A., Bastida, R., Valencia, L., Macias, A. and Lodeiro, C. (2006) Metal complexes with two tri-aza, tri-oxa pendant-armed macrocyclic ligands: Synthesis, characterization, crystal structures and fluorescence studies. Inorganica Chimica Acta, 359, 2383-2394. doi:10.1016/j.ica.2005.12.055
- Faniran, J. A., Patel, K. S. and Bailar, J. C. (1974) Infrared spectra of N,N’-bis(salicylidene)-1,1-(dimethyl)- ethylenediamine and its metal complexes. Journal of Inorganic and Nuclear Chemistry, 36, 1547-1551. doi:10.1016/0022-1902(74)80621-4
- Yano, S., Takizawa, S., Sugita, H., Takahashi, T., Shioi, H., Tsubomura, T. and Yoshikawa, S. (1985) Reactions of metal complexes with carbohydrates: Synthesis and characterization of novel nickel(II) complexes containing glycosylamines derived from a monosaccharide and a diamine. An X-ray crystallographic study of (ethylenediamine) {N-(2-aminoethyl)-d-fructopyranosylamine} nickel(II)-Cl2-CH3OH. Carbohydrate Research, 142, 179-193. doi:10.1016/0008-6215(85)85021-7
- Bian, H.D., Xu, J.Y., Gu, W., Yan, S.P., Liao, D.Z., Jiang, Z.H. and Cheng, P. (2003) Synthesis, structure and properties of terephthalate-bridged copper(II) polymeric complex with zigzag chain. Inorganic Chemistry Communications, 6, 573-576.
- Mohamed, G.G., Omar, M.M. and Hindy, A.M. (2006) Metal complexes of Schiff basess, preparation, characterization and biological activity. Turkish journal of chemistry, 30, 361-382.
- El-Ayaan, U., El-Metwally, N.M., Youssef, M.M. and El Bialy, S.A.A. (2007) Perchlorate mixed-ligand copper(II) complexes of β-diketone and ethylene diamine derivatives: Thermal, spectroscopic and biochemical studies. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 68, 1278-1286. doi:10.1016/j.saa.2007.02.011
- Surati, K.R. and Thake, B.T. (2010) Synthesis, spectral, crystallography and thermal investigations of novel Schiff base complexes of manganese(III) derived from heterocyclic β-diketone with aromatic and aliphatic diamine. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 75, 235-242. doi:10.1016/j.saa.2009.10.018
- Bharty, M.K., Srivastava, A.K., Dulwere, R., Butcher, R.J. and Singh, N.K. (2011) synthesis, spectral and X-ray structural studies of Ni(II) complexes of N’-acylhydrazine carbodithioic acid esters containing ethylenediamine or o-phenanthroline as coligands. Polyhedron, 30, 990-996. doi:10.1016/j.poly.2010.12.043
- Ghazy, S.E., Kabil, M.A., Shallaby, A.M. and Ammar, N.S. (2001) Flotation-separation of the pollutant species of chromium, cadmium and lead from aqueous solutions and natural waters. Indian Journal of Chemical Technology, 8, 211-218. doi:10.1021/ac00232a002
- Leyden, D.E. and Wegschider, W. (1981) Preconcentration for trace element determination in aqueous samples. Analytical Chemistry, 53, 1059-1065.
- Zouboulis, A.I., Lazaridis, N.K. and Zamboulis, D. (1994) Powdered activated carbon separation from water by foam floation, Separation Science and Technology, 29, 385-400. doi:10.1080/01496399408002490
- Lajunen, L.H.J. (1991) Spectrochemical analysis by atomic absorption and emission. Royal Society of Chemistry, Cambridge.
- Vogel, A.I. (1962) Quantitative inorganic analysis includeing elemental instrumental analysis. 2nd Edition, Longmans, London.
- Sari, N., Arslan, S., Logoglu, E. and Sakiyan, I. (2003) Antibacterial activities of some amino acid Schiff bases. Journal of Animal Science, 16, 283.
- Batley, G.E. and Gardner, D. (1977) Sampling and storage of natural waters for trace metal analysis. Water Research, 11, 745-756. doi:10.1016/0043-1354(77)90042-2
- Masoud, M.S., Hindawy, A.M. and Soayed, A.S. (1991) Structural chemistry of some new azo complexes. Transition Metal Chemistry, 16, 372-376. doi:10.1007/BF01024086
- Coats, A.W. and Redfern, J.P. (1964) Kinetic parameters from thermogravimetric data. Nature, 201, 68-79. doi:10.1038/201068a0
- Duraiswamy, S, Michael, R.E., Matthias, Z. and Karuppannan, N. (2007) Hydrolytic cleavage of Schiff bases by [RuCl2(DMSO)4]. polydedron, 26, 4314-4320.
NOTES
*Corresponding author.

