Journal of Surface Engineered Materials and Advanced Technology
Vol.3 No.3(2013), Article ID:34216,6 pages DOI:10.4236/jsemat.2013.33021
Thermal Behavior and UV Properties of Organomodified Kaolin Oleochemically Derived from Rubber Seed Oils (Hevea brasiliensis) and Tea Seed Oils (Camellia sinensis)
![]()
1National Engineering Design Development Institute, Nnewi, Nigeria; 2Department of Mechanical Engineering, Nnamdi Azikiwe University, Awka, Nigeria; 3National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, India.
Email: *edumgbemena@yahoo.com, *mgbemenachinedum@neddinaseni.org
Copyright © 2013 Chinedum Ogonna Mgbemena et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 12th, 2013; revised February 12th, 2013; accepted May 1st, 2013
Keywords: Kaolin; Rubber Seed Oil; Tea Seed Oil; Characterization; Ultraviolet
ABSTRACT
Kaolin was modified using a chemical complex of hydrazine hydrate and oleochemical sodium salts derived from rubber seed oil (SRSO) and tea seed oil (STSO) respectively. Characterization of the pristine kaolin and the modified kaolins were performed using Scanning Electron Microscopy (SEM), Simultaneous Thermogravimetric/Differential Thermal Analysis (TG/DTA) and UV Spectrophotometry. TG/DTA revealed that the incorporation of the oleochemical salts enhanced thermal decomposition of kaolin into metakaolin. Ultraviolet spectrophotometric studies conducted on the modified kaolin show for the first time that the SRSO-modified kaolin and STSO-modified kaolin have a peak absorbance wavelengths of 312.72 nm and 314.26 nm respectively. This shows that the modified kaolin is a promising candidate for sunscreen applications.
1. Introduction
Kaolin is ubiquitous and has wide range of applications, in various fields of science, due to the propensity with which they can be chemically and physically modified to suit practical technological needs. They are used in ceramics, medicine, paper coatings, as a food additive, in toothpaste, as a light diffusing material in white incandescent light bulbs, and in cosmetics. It is generally the main component in porcelain. It is also used in paint production to extend titanium dioxide (TiO2) and modify gloss levels; in rubber for semi-reinforcing properties; and in adhesives to modify rheology [1]. Kaolin can be consumed to suppress hunger and for pleasure in some parts of the world, a practice known as geophagy [2].
The empirical formula for kaolin is Al2Si2O5(OH)4 and the theoretical chemical composition is SiO2, 46.54%; Al2O3, 39.50%; and H2O, 13.96%. Kaolin has a 1:1 sheet structure comprising of tetrahedral and octahedral sheets. It is made up of tiny, thin, pseudo hexagonal, flexible sheets of triclinic crystal with a diameter of 0.2 - 12 μm. It has a density of 2.1 - 2.6 g/cm3. The cation exchange capacity of kaolin is in the order of 2 - 10 meq/100 g, depending on the particle size, but the rate of the exchange reaction is rapid, almost instantaneous. Upon heating, kaolinite starts to lose water at approximately 400˚C, and the dehydration approaches completeness at approximately 525˚C. The dehydration depends on the particle size and crystallinity [3].
The research of intercalation of organic molecules into the interlayer space of clay minerals started in the 1920s, after the introduction of X-ray diffraction in 1913 [4].
The most known compounds that intercalate directly with kaolin are polar organic molecules such as fatty acid salts, hydrazine hydrate, dimethylsulphoxide (DMSO), formamide, potassium acetate and urea. They are reported in the literature as having the ability to disrupt the interlayer bonding between the adjacent siloxane and hydroxy aluminum surfaces and to penetrate the interlayer space to form a complex by hydrogen bonding to both surfaces [5].
Other compounds can also be introduced in the kaolinite layers by the displacement of a previously intercalated compound [6-9]. Detailed studies on the intercalation of kaolin and highly polar organic molecules such as amides and sulphoxides were reported [10-12].
The use of organomodifiers such as octyl amine, octadecyl amine, dodecyl amine, dimethyl distearyl ammonium bromide, dimethyl benzyl hydrogenated tallow quaternary ammonium, dimethyl dehydrogenated tallow quaternary ammonium, and methyl tallow bis-2-hydroxy ethyl quaternary ammonium has been reported [4].
Several reports have been made on the suitability of oleochemical sources of rubber seed oil and tea seed oil to be used as precursors for organic modification of kaolin and the following characterization studies: Differential Thermal Analysis (DTA), Fourier Transform Infrared Spectroscopy (FTIR) and X-ray diffraction (XRD) performed on the modified kaolin [13-16].
Until now, there are not many reports on the thermal behavior and UV properties of modified kaolin from oleochemical sources.
This paper discusses the results obtained from morphological studies using SEM; thermal analysis and possible degradation mechanism of organomodified kaolin from oleochemically derived Sodium salts rubber Seed oil and tea seed oil obtained by simultaneous TG/DTA and the UV properties of the modified kaolin obtained from UV-vis-spectrophotometer. In this article, the morphology and thermal behavior of the pristine kaolin were also discussed.
2. Experimental
2.1. Materials
Kaolin (BCK grade) used in this study was obtained from M/s. English Indian Clays Ltd, Veli, Thiruvananthapuram, Kerala, India. Rubber Seed Oil (RSO) and Tea Seed Oil (TSO) were obtained from National Institute for Interdisciplinary Science and Technology, India. Sodium hydroxide (MERCK), hydrazine hydrate (FINNAR) were obtained from local suppliers. The kaolin, RSO and TSO has the following chemical composition determined by chemical analysis, as reported in Table 1.
2.1.1. The Synthesis of SRSO-Modified Kaolin
Kaolin was slowly added to a mixture-containing of SRSO and hydrazine hydrate in the ratio 4.9:1:3.5 with vigorous stirring at 20˚C. The mixture was homogenized well using Art-MICCRA D-8 (Germany) homogenizer, and the sample was dried using a freeze drier [HetroTrap-CT60e, JOUAN]. The sample was designated as K2.
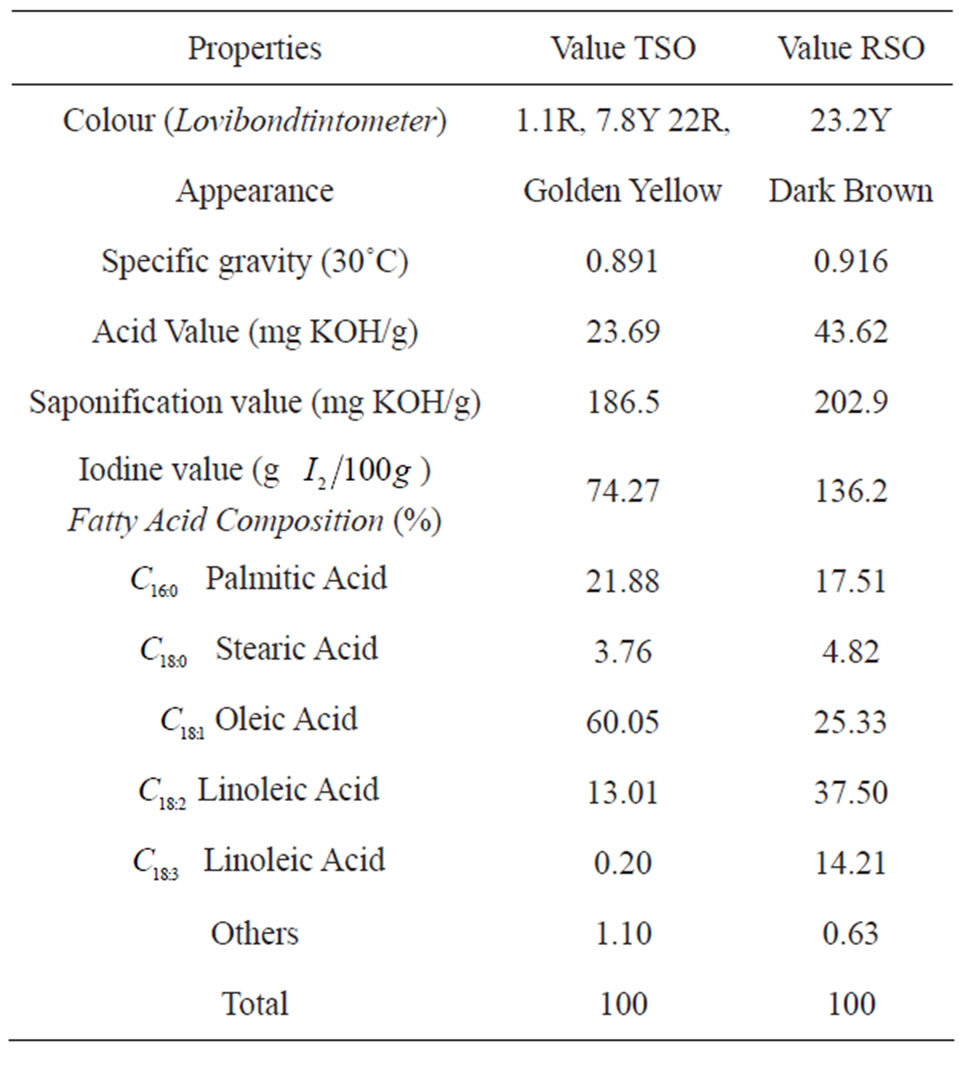
Table 1. Physicochemical characteristics and fatty acid profiles of TSO and RSO.
2.1.2. Conversion of TSO into its Sodium Salt
Sodium salt of tea seed oil (STSO) was synthesized by reacting 28 mL of TSO with 27 mL of 20% NaOH in an ice bath with constant stirring for 12 hours and kept for 1 day to allow for curing to take place. The pH of the resulting solution was maintained at 8 - 9. STSO was then introduced into a separating funnel and washed with water to remove excess base. This was then dried in a hot air oven to remove residual moisture and powdered.
2.1.3. The Synthesis of STSO-Modified Kaolin
Kaolin was slowly added to a mixture-containing of STSO and hydrazine hydrate in the ratio 4.9:1:3.5 with vigorous stirring at 20˚C. The mixture was homogenized well using Art-MICCRA D-8 (Germany) homogenizer and the sample was dried using a freeze drier [HetroTrap-CT60e, JOUAN]. The sample was designated as K4.
2.2. Characterization
2.2.1. Scanning Electron Microscopy
The pristine and organomodified kaolin were examined using JEOL JSM-5600 LV Scanning Electron Microscope at a magnification of 15,000 ×.
2.2.2. Thermogravimetric/Differential Thermal Analysis
The dehydration process and weight losses of pristine and organomodified kaolins were studied by simultaneous Thermogravimetric/Differential Thermal analysis (TG/DTA) in which the clays are subjected to a temperature scan from 50.00˚C to 995.00˚C at the rate of 10.00˚C/min in the presence of oxygen at 20.0 ml/min using the Perkin Elmer STA 6000 for Simultaneous Thermal Analyzer.
2.2.3. UV-Vis-Spectrophotometric Studies
The spectrophotometer (SHIMADZU UV-1601, Tokyo, Japan) was used for the UV-visible spectrophotometric studies.
3. Results and Discussions
3.1. Scanning Electron Microscopy
Figures 1-3 show the SEM micrographs of the pristine kaolin, the SRSO-modified kaolin and the STSO-modified kaolin at a magnification of 15,000 ×. In Figure 1, the pristine kaolin SEM micrograph revealed the platelet hexagonal structure of the kaolinite crystals.
In Figure 2 the SRSO-modified kaolin has a compact platelet morphology with delaminated layers which shows the presence of organic material (organomodification) in the kaolin. The STSO-modified morphology in Figure 3 clearly shows the presence of organic material in the kaolin matrix. The STSO-modified kaolin has farinaceousdelaminated layers indicating an intercalation with organic material.
3.2. Thermogravimetric/Differential Thermal Analysis
Two major decomposition steps can be deduced from the TGA plot of Kaolinites (see Stages 1 and 2 below):
For the SRSO-modified kaolin, the visible two stage decomposition occurs at 235˚C - 580˚C for the first stage and 705˚C - 1000˚C for the second stage. Similarly, in the STSO-modified kaolinites, the first stage decomposition occurs at temperatures 240˚C - 342˚C while the second stage occurs at 585˚C - 1000˚C. It was observed that the thermal decomposition of kaolin is lowered on organomodification. The decomposition rate of the STSO-modified kaolin was faster compared to the pristine and the SRSO-modified; this may be due to the low specific gravity of the Tea seed oil. Figures 4-7 shows the TG/DTA plots of the pristine kaolin, the SRSOmodified Kaolin and the STSO-modified Kaolin. The DTA profile for the pristine kaolin shows the first endo-
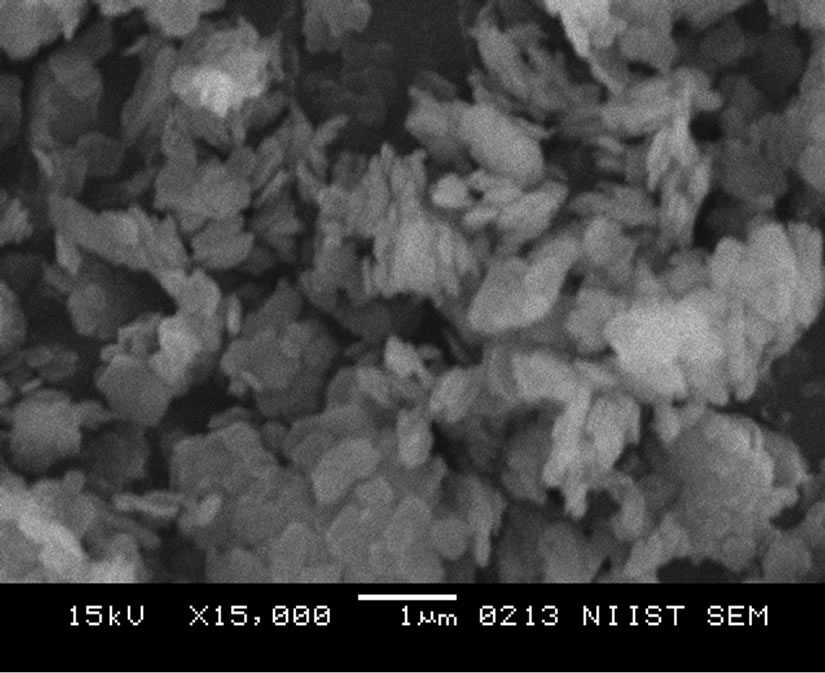
Figure 1. Unmodified kaolin at a magnification of × 15,000.
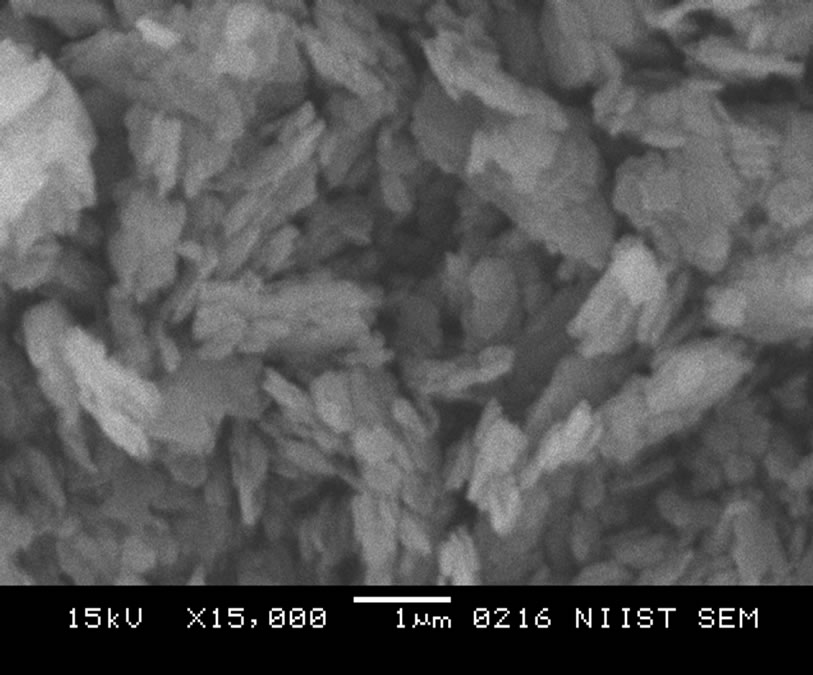
Figure 2. SRSO-modified kaolin at a magnification of × 15,000.
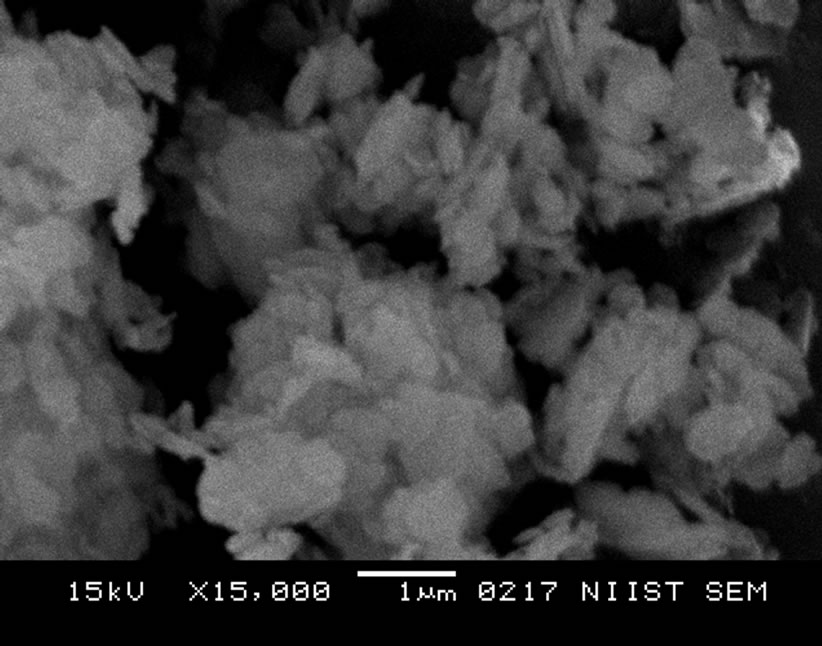
Figure 3. STSO-modified kaolin at a magnification of × 15,000.
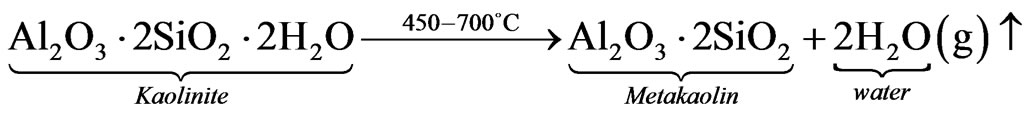 Stage 1
Stage 1
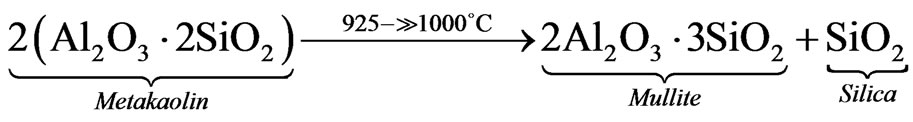 Stage 2
Stage 2
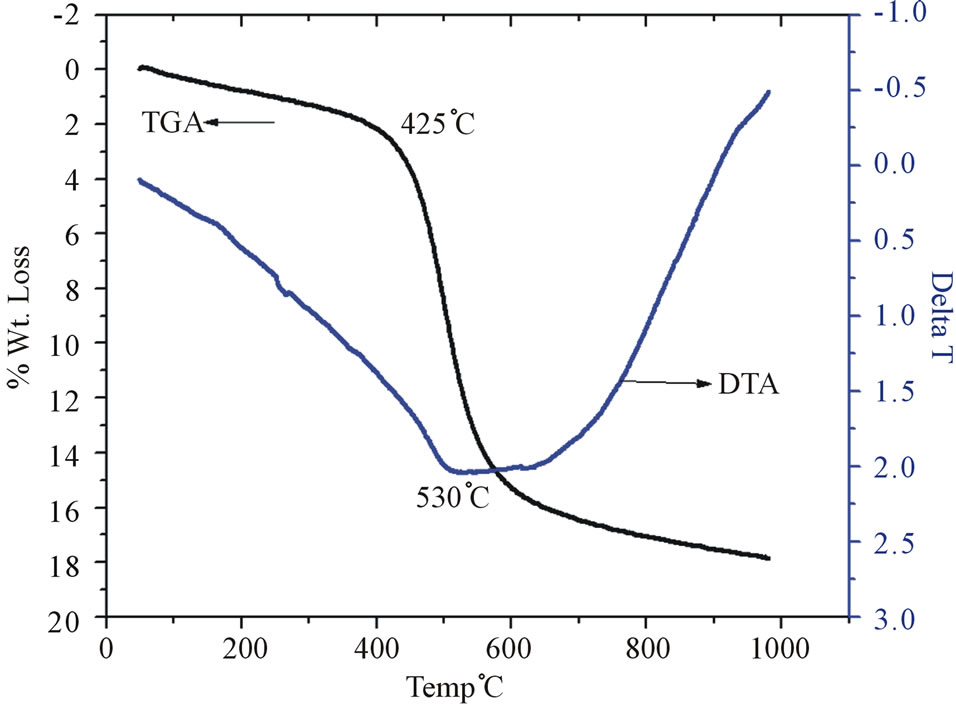
Figure 4. TG/DTA plot for unmodified kaolin.
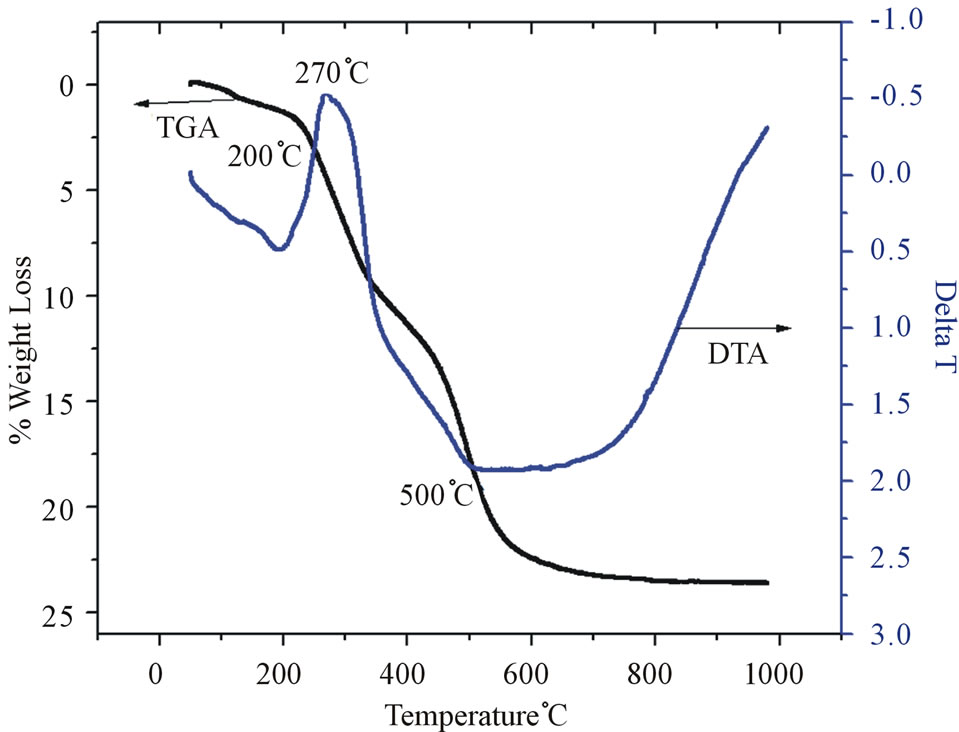
Figure 5. TG/DTA plot for SRSO-modified kaolin.
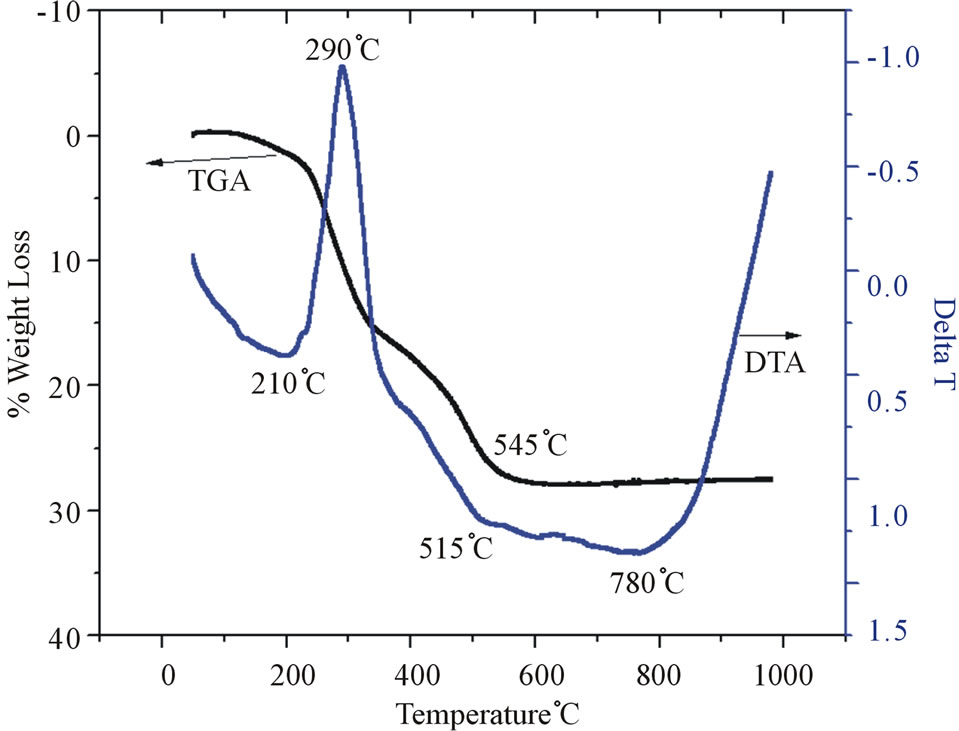
Figure 6. TG/DTA plot for STSO-modified kaolin.
thermic mass loss at 50˚C - 200˚C due to moisture loss. The major endothermic mass loss of the pristine kaolin was observed in the temperature ranges of 450˚C -
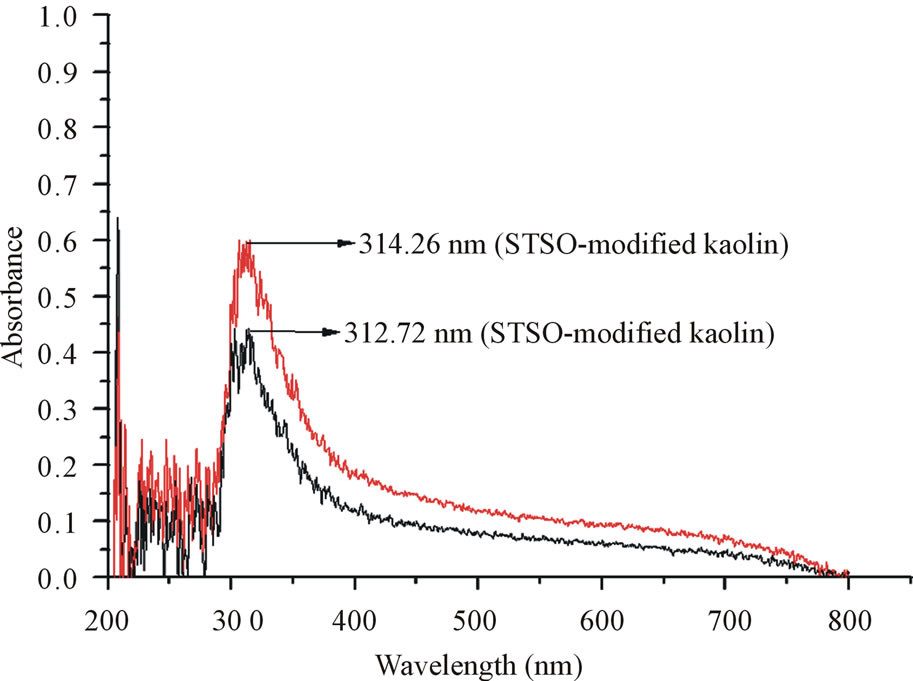
Figure 7. UV Spectroscopy of the organomodified Kaolin.
550˚C. The minimum weight loss occurs at 530˚C; this agrees with the result of a previous work by Sukumar and Menon [13]. The endotherm temperature of 530˚C represents the loss of structural hydroxyls by dehydroxylation (or alternatively, dehydration) producing highly reactive metakaolin. Dehydroxylation process continued up to 900˚C which may be attributed to oxolation of the metakaolin [17,18]. The exothermic peak at around 1000˚C is due to the crystallization of the amorphous metakaolinite [19-22]. In Figure 5, the DTA profile for the SRSOmodified kaolin shows an endotherm in the temperature range 500˚C. This lower exothermic temperature is due to the presence of an impurity phase attributed to the SRSO. The peak at 270˚C is attributed to the combustion of the hydrocarbon part of SRSO and this proves the existence of SRSO in the interlayer spacing of the kaolin. The low endotherm temperature of 500˚C represents the loss of structural hydroxyls by dehydroxylation producing highly reactive metakaolin. The exothermic peak at around 1000˚C is due to the crystallization of the amorphous metakaolinite.
In Figure 6, the DTA profile for the STSO-modified kaolin shows an endotherm in the temperature range 515˚C. This lower exothermic temperature is due to the presence of an impurity phase attributed to the STSO. The peak at 290˚C is attributed to the combustion of the hydrocarbon part of STSO and this proves the existence of STSO in the interlayer spacing of the kaolin. The low endotherm temperature of 515˚C represents the loss of structural hydroxyls by dehydroxylation producing highly reactive metakaolin. The exothermic peak at around 1000˚C is due to the crystallization of the amorphous metakaolinite.
3.3. UV-Vis Spectrophotometric Studies
The UV studies were conducted by using the pristine kaolin dissolved in distilled water as the baseline reference. The SRSO-modified and STSO-modified kaolin samples were then studied to know the absorbance within the UV range of 200 - 800 nm. The SRSO-modified kaolin has a peak absorption (λmax) wavelength of 312.72 nm while the STSO-modified has a peak absorption (λmax) wavelength of 314.26 nm. The modified kaolin can be used in sunscreens as a UV absorber. They can absorb Ultaviolet B (UVB) which is the medium range from 315 - 280 nm with energy per photon values of 3.94 - 4.43 eV [23].
4. Conclusions
The SEM morphologies of the modified kaolin show that there was intercalation of the organic substance in the kaolinite layers. The dehydroxylation of the kaolin was enhanced by modification to yield metakaolin. The enhancement in the decomposition time and temperature of the modified kaolinite could be as a result of decrease in the electrostatic attraction between the lamellae due to intercalation. An industrial treatment of kaolin with fatty acid salts is recommended. This will enhance the thermal decomposition of the kaolin and optimum yield of metakaolin which is classified as a new generation of supplementary cementitous material. Metakaolin is usually produced by thermal treatments, i.e., calcination of kaolin clays within a definite temperature range. Metakaolin is unique in that it is neither the by-product of an industrial process nor is it entirely natural; it is derived from a naturally occurring mineral, and it is manufactured specifically for cementing applications [24].
The results from this study also show that the modified kaolin is a potential candidate for sunscreen applications as a UV absorbance. The modified kaolin has the advantage of being non-toxic and from oleochemical sources.
5. Acknowledgements
One of the authors, Chinedum O. Mgbemena is grateful to Centre for International Co-operation in Science (CICS) and DST, Government of India for RTFDCS Fellowship. Thanks to National Institute for Interdisciplinary Science and Technology, (CSIR-NIIST), Trivandrum, India for generously providing materials and facilities for this work.
REFERENCES
- P. A. Ciullo, “Industrial Minerals and Their Uses: A Handbook and Formulary,” Noyes Publications, Park Ridge, 1996.
- G. N. Callahan, “Eating Dirt,” Emerging Infectious Diseases,” 2003.
- R. E. Grim, “Clay Mineralogy,” McGraw-Hill, Inc., New York, 1968, p. 596
- D. Merinska, Z. Malac, M. Pospisil, Z. Weiss, M. Chmielova, P. Capkova and J. Simonik, “Polymer/Clay Nanocomposites Based on MMT/ODA Intercalates,” Composite Interfaces, Vol. 9, No. 6, 2002, pp. 529-540. doi:10.1163/15685540260494100
- M. J. Wilson, “A Handbook of Determinative Methods in Clay Mineralogy,” Blackie & Son Ltd., London, 1987.
- Y. Komori, Y. Sugahara and K. Kuroda, “Direct Intercalation of Poly(vinylpyrrolidone) into Kaolinite by a Refined Guest Displacement Method,” Chemistry of Materials, Vol. 11, No. 1, 1999, pp. 3-6. doi:10.1021/cm9804721
- W. N. Martens, R. L. Frost, J. Kristof and E. Horvath, “Modification of Kaolinite Surfaces through Intercalation with Deuterated Dimethylsulfoxide,” Journal of Physical Chemistry B, Vol. 106, No. 16, 2002, pp. 4162-4171. doi:10.1021/jp0130113
- E. Horvath, J. Kristof, R. L. Frost, E. Jakab, E. Mako ,V. Vagvolgyi, “Identification of Superactive Centers in Thermally Treated Formamide-Intercalated Kaolinite,” Journal of Colloid and Interface Science, Vol. 289, No. 1, 2005, pp. 132-138. doi:10.1016/j.jcis.2005.03.059
- R. L. Frost, J. Kristof, E. Horvath and J. T. Kloprogge, “Modification of Kaolinite Surfaces through Intercalation with Potassium Acetate, II,” Journal of Colloid and Interface Science, Vol. 214, No. 1, 1999, pp. 109-117. doi:10.1006/jcis.1999.6177
- J. C. Dai and J. T. Huang, “Surface Modification of Clays and Clay-Rubber Composite,” Applied Clay Science, Vol. 15, No. 1-2, 1999, pp. 51-65. doi:10.1016/S0169-1317(99)00020-4
- M. Lipsicas, R. Raythatha, R. F. Giese and P. M. Costanzo, “Molecular Motions, Surface Interactions, and Stacking Disorder in Kaolinite Intercalates,” Clays and Clay Minerals, Vol. 34, No. 6, 1986, pp. 635-644. doi:10.1346/CCMN.1986.0340603
- M. Raupach, P. F. Barron and J. G. Thompson, “Nuclear Magnetic Resonance, Infrared, and X-Ray Powder Diffraction Study of Dimethylsulfoxide and Dimethylselenoxide Intercalates with Kaolinite,” Clays and Clay Minerals, Vol. 35, No. 3, 1987, pp. 208-219. doi:10.1346/CCMN.1987.0350307
- S. Rugmini and A. R. R. Menon, “Organomodified Kaolin as Filler for Natural Rubber,” Journal of Applied Polymer Science, Vol. 107, No. 6, 2008, pp. 3476-3483. doi:10.1002/app.27469
- L. E. Yahaya, K. O. Adebowale and A. R. R. Menon, “Mechanical Properties of Organomodified Kaolin/Natural Rubber Vulcanizates,” Applied Clay Science, 46, No. 3, 2009, pp. 283-288. doi:10.1016/j.clay.2009.08.018
- L. E. Yahaya, K. O. Adebowale, B. I. Olu-Owolabi, A. R. R. Menon, R. Sukumar and J. Chameswary, “Natural Rubber/Organoclay Nanocomposite from Tea (Camellia sinesis) Seed Oil Derivative,” American Journal of Materials Science, Vol. 2, No. 2, 2012, pp. 1-5.
- L. E. Yahaya, K. O. Adebowale, B. I. Olu-Owolabi and A. R. R. Menon, “Compositional Analysis of Tea (Camellia sinensis) Seed Oil and its Applications,” International Journal of Research in Chemistry and Environment, Vol. 1, No. 2, 2011, pp. 153-158.
- M. Bellotto, A. Gualtieri and G. Artioli, “Kinetic Study of the Kaolinite Mullite Reaction Sequence. Part I: Kaolinite Dehydroxylation,” Physics and Chemistry of Minerals, Vol. 22, No. 4, 1995, pp. 207-214. doi:10.1007/BF00202253
- A. Gualtieri, M. Bellotto and G. Artioli, “Kinetic Study of the Kaolinite Mullite Reaction Sequence. Part 2: Mullite Formation,” Physics and Chemistry of Minerals, Vol. 22, No. 4, 1995, pp. 215-222. doi:10.1007/BF00202254
- E. Badogiannis, G. Kakali and S. Tsivilis, “Metakaolin as Supplementary Cementitious Material—Optimization of Kaolin to Metakaolin Conversion,” Journal of Thermal Analysis and Calorimetry, Vol. 81, No. 2, 2005, pp. 457- 462. doi:10.1007/s10973-005-0806-3
- A. Shvarzman, K. Kovler and G. Grader, “The Effect of Dehydroxylation/Amorphization Degree on Pozzolanic Activity of Kaolinite,” Cement and Concrete Research, Vol. 33, No. 3, 2003, pp. 405-416. doi:10.1016/S0008-8846(02)00975-4
- H. Rahier, B. Van Mele and M. Biesemans, “LowTemperature Synthesized Aluminosilicate Glasses. 2. Rheological Transformations during Low-Temperature Cure and High-Temperature Properties of a Model Compound,” Journal of Materials Science, Vol. 31, No. 1, 1996, pp. 80-85. doi:10.1007/BF00355129
- D. W. Breck, “Zeolite Molecular Sieves,” Wiley-Interscience, New York, 1974, pp. 314-315.
- “ISO 21348 Process for Determining Solar Irradiances”. http://www.spacewx.com/pdf/SET_21348_2004.pdf
- B. R. Ilić, A. A. Mitrović and L. R. Miličić, “Thermal Treatment of Kaolin Clay to Obtain Metakaolin,” Hemijska Industrija, Vol. 64, No. 4, 2010, pp. 351-356.
NOTES
*Corresponding author.

