International Journal of Organic Chemistry
Vol. 2 No. 3 (2012) , Article ID: 22733 , 5 pages DOI:10.4236/ijoc.2012.23026
Adsorbent for Arsenic(V) Removal Synthesized by Radiation-Induced Graft Polymerization onto Nonwoven Cotton Fabric
1Environment and Industrial Materials Research Division, Quantum Beam Science Directorate, Japan Atomic Energy Agency, Takasaki, Japan
2ERH Techno Research Co., Ltd., Takasaki, Japan
Email: *hoshina.hiroyuki@jaea.go.jp
Received July 29, 2012; revised September 2, 2012; accepted September 10, 2012
Keywords: Arsenic Removal; Cotton; Radiation-Induced Graft Polymerization; Phosphoric Acid; Zirconium
ABSTRACT
A fibrous adsorbent for arsenic (As) removal was synthesized with nonwoven cotton fabric as a trunk polymer. 2-hydroxyethyl methacrylate phosphoric acid monomer which composed of phosphoric acid mono (50%) and di (50%) ethyl methacrylate ester was introduced with radiation-induced graft polymerization onto nonwoven cotton fabric. The degree of grafting of 130% was obtained at irradiation dose of 20 kGy with 5% of monomer solution for 2 hours reaction time at 40˚C reaction temperature. After the grafted material was contacted with 10 mmol/L of zirconium (Zr) solution at pH 1, 0.38 mmol/g of Zr was loaded on phosphoric units as a functional group for As(V) removal. The resulting adsorbent was evaluated by column mode adsorption with 1 mg/L of As(V) solution at various pH with space velocity 200 h–1. The maximum capacity of As(V) adsorption was 0.1 mmol/g at pH 2.
1. Introduction
The impact of water pollution by toxic metals on human health remains a significant problem throughout the world. Arsenic (As), which is one of the most common causes of water pollution, exists in high concentrations in natural water such as rivers, hot springs, groundwater and drainage from abandoned mines [1-4]. To solve the problem, a coagulation and ion-exchange of granular resin have been extensively used for removal of As(V) from water [5-6]. However, a large amount of waste is produced by water treatment with coagulation, and it is difficult to remove As(V) to a low concentration rapidly with these techniques. Hence, a new adsorbent which could effectively remove As(V) from water is needed.
Zirconium (Zr) has been used for adsorption of As(V) as a functional group [7,8]. The reason is Zr(V) has a particular affinity for As(V). But Zr(V) could not be directly introduced onto polymeric materials. Phosphoric group has high affinity against metal such as Fe, Mo, Co and Zr. Especially, Zr(V) strongly binds with phosphate at high concentration of nitric acid. Therefore, phosphorric group was introduced onto polymeric materials to load Zr(V). Then the strongly bound complex of phosphoric-Zr(V) shows high adsorption capacity of As(V), which means Zr(V) plays a role of intermediary in the adsorption process.
Radiation-induced graft polymerization (RIGP) is a useful method to introduce a functional group onto polymeric materials. The advantages of this method are rapidly creating radicals in a trunk material by irradiation without any chemical reagents and easily introducing functional groups onto a trunk material without signifycant changes in the properties of the trunk material [9, 10]. The fibrous adsorbent, which was synthesized by RIGP, can rapidly remove and effectively recover metal from water. Therefore, many types of adsorbent have been produced with RIGP. Normally these adsorbents were synthesized by using petroleum materials such as polyethylene and polypropylene as a trunk material [11-13]. Graft polymerization using cotton, which one of plant-based material, as a trunk polymer is possible to decrease petroleum resources dependence and contribute to conserving resources.
In this study, the Zr-type adsorbent for As(V) removal was synthesized by RIGP with a phosphoric monomer onto a nonwoven cotton fabric and was subsequently modified with Zr(IV) loading on phosphoric units. The adsorption behavior of the Zr-type adsorbent with As(V) was investigated by column mode adsorption.
2. Experimental
2.1. Materials
A nonwoven cotton fabric purchased from Marusan Industry Co., Ltd. was used as a trunk polymer. Phosphoric acid monomer (2-hydroxyethyll methacrylate phosphoric acid; Kyoeisha Co.) was used for grafting monomer which was composed of the mixture of mono (50%) and diester (50%) as shown in Figure 1. Zirconium sulfate tetrahydrate and Di-sodium hydrogen arsenate heptahydrate were supplied by Kanto Chemical Co., INC. All other chemicals and solvents were used without further purification.
2.2. Graft Polymerization
The nonwoven cotton fabrics were cut into 3 cm × 5 cm and were packed in a polyethylene bag under nitrogen atmosphere, which were irradiated with electron beam at beam energy of 2 MV and current of 3 mA under dry-ice temperature. The irradiated fabrics were placed into a glass ampoule and were evacuated. The aqueous emulsion of phosphoric acid monomer was transferred into the ampoule. The reaction temperature was maintained in water bath at 40˚C and 50˚C. After graft polymerization, the grafted materials were washed several times with pure water and dried in vacuum oven at 30˚C. The degree of grafting (Dg) was calculated by the following equation;
![]()
where W0 and W1 are the weights of nonwoven cotton fabrics before and after graft polymerization, respectively.
2.3. Loading of Zirconium on Phosphoric Units
Zr(IV) was loaded on phosphoric units by the contacting of Zr(IV) solution with a column. The grafted materials were packed into the column with an internal diameter of 1 cm. The height of grafted materials in the column was 0.3 cm. The Zr(IV) solution was prepared by dissolving Zirconium sulfate tetrahydrate in pure water. The pH of Zr(IV) solution was adjusted to pH 1 and pH 2 by addition of nitric acid. The Zr(IV) solution was delivered up-flow to
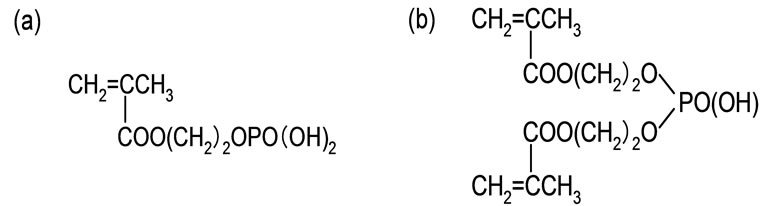
Figure 1. Chemical structure of phosphoric acid monomer: (a) mono ester; (b) diester.
the column at flow rate of space velocity (SV) 200 h-1 using a peristaltic pump [Advantec, AP-1600]. After Zr(IV) loading, the adsorbent in the column was washed with pure water.
2.4. Arsenic Adsorption
To evaluate the adsorption performance for As(V), the adsorption test was investigated by column mode test. The solution of As(V) was prepared by dissolving disodium hydrogen arsenate heptahydrate in pure water. The pH of As(V) solution was adjusted with nitric acid and ammonia solution. These solutions were pumped into the column at various flow rates and were collected by the fraction collector. The concentration of As(V) was determined by induced coupled plasma atomic emission spectroscopy [ICP-AES, Perkin Elmer Inc.,Optimaa 4300 DV]. The breakthrough point was defined as the bed volume (BV) of feed solution at As(V) concentration in the effluents reached 5% of that in the feed solution. The characteristic of breakthrough was observed by plotting C/C0 which was the value of effluent dived by the feeding concentration against BV, where C0 and C are the concentration of As(V) in the feeding solution and in the effluent, respectively.
3. Result and Discussion
3.1. Graft Polymerization
In order to minimize the mechanical damage of cotton fabric from irradiation, the graft polymerization of phosphoric acid monomer was carried out at irradiation dose of 20 kGy. Figure 2 shows the effect of reaction time on Dg with monomer concentration of 5 and 10% at 40˚C. The Dg increased with increment of the reaction time. And the Dg becomes 2 times higher with the increase of monomer concentration from 5% to 10%. The Dg of 130% was obtained at monomer concentration of 5% for 2 hours which is satisfied the requirements for preparing As(V) adsorbent. Therefore, it was concluded that the
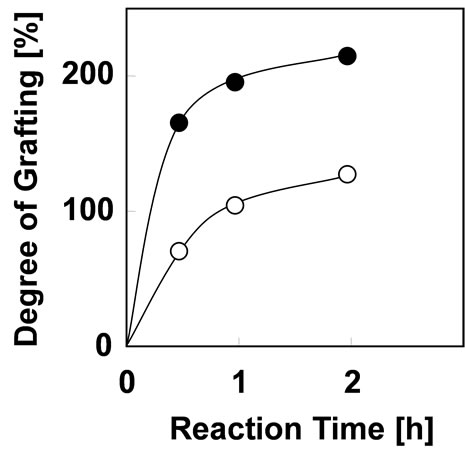
Figure 2. Effect of reaction time on Dg with monomer concentration of (○) 5% and (●) 10% at 40˚C.
optimum graft condition of phosphoric acid monomer is monomer concentration of 5% on the reaction time of 2 hours at reaction temperature of 40˚C. In comparison with a graft polymerization using polyethylene as a trunk polymer [14], cotton-grafting could be reduce monomer concentration, reaction time, reaction temperature and irradiation dose, which indicates that the cotton-grafting is effective and economical to introduce a phosphoric acid monomer onto trunk polymer. Surface of cotton fabric was observed with scanning electron microscope [SEMEDX Type N, HITACHI]. SEM photographs of cotton fabric before and after grafting were shown in Figure 3. After grafting for 2 hours, the diameter increased from 12 μm to 20 μm, and the damage from grafting did not appear on surface of the cotton fabric.
3.2. Loading of Zirconium on Phosphoric Units
The Zr(IV) loading on phosphoric units was carried out by pumping 10 mmol/L of Zr(IV) solution into the grafted material-packed column at SV200 h–1. Figure 4 shows the characterization of Zr(IV) loading. Almost of all Zr(IV) was loaded on phosphoric units around 10 BV. As a result, 0.38 mmol/g of Zr(IV) was loaded on the grafted material by 50 BV feeding at pH 1. The maximum Zr(IV) density at pH 2 was 0.31 mmol/g, which is lower than the value obtained at pH 1. Hence, the Zr(IV) loading was performed at pH 1.
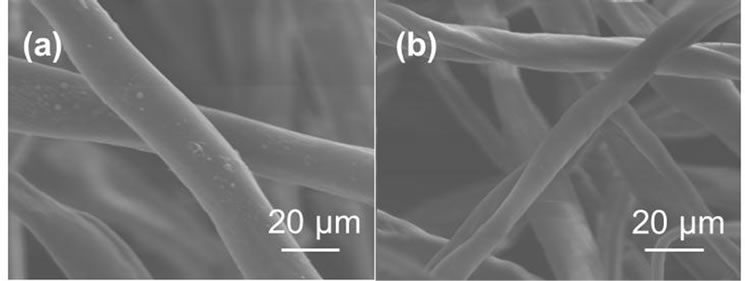
Figure 3. SEM photographs of (a) trunk polymer and (b) grafted polymer of 130%.
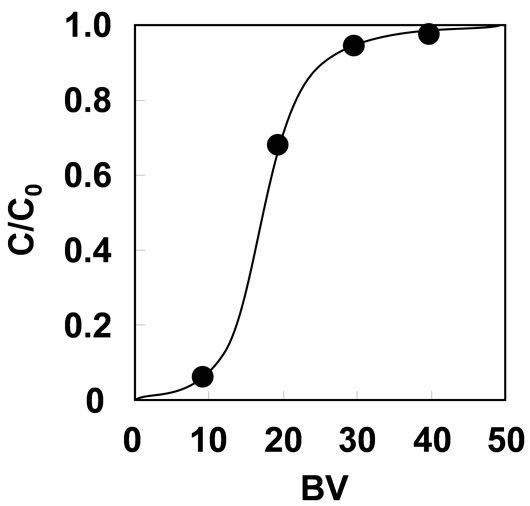
Figure 4. Zirconium(IV) loading on phosphoric units with 10 mmol/L of Zr(IV) solution on SV200 h–1 at pH 1.
3.3. Column Mode Adsorption of Arsenic
Column mode adsorption of As(V) was performed with 1 mg/L of As(V) solution. The breakthrough curves of As(V) adsorption at various pH with SV 200 h–1 is shown in Figure 5. The adsorbent can adsorb As(V) across a wide range of pH values. The BV at breakthrough point were 870, 1420, 900, 550, 330 and 270 at pH 1, 2, 3, 5, 7 and 9, respectively. The maximum capacity of As(V) adsorption was 0.1 mmol/g at pH 2. At pH range of higher than 3, As(V) capacity of the adsorbent signifycantly decreased with increment of pH, and also decreased slightly at pH 1. Therefore, the effect of flow rate on As(V) adsorption was investigated at pH 2.
Figure 6 shows the breakthrough curves of As(V) adsorption with As(V) solution of 1 mg/L at SV 200 and 1000 h–1. The adsorption capacity for As(V) was 0.1 mmol/g with either flow rate. Increasing the flow rate up to SV 1000 h-1 did not have significant effect on the adsorption capacity of As(V). In general, the granular resin adsorbent is effective for As(V) adsorption at relatively slow flow rate around SV 10 h–1[7]. It means that the fibrous adsorbent, which was synthesized by RIGP, can adsorb As(V) 100 times faster than granular resin.
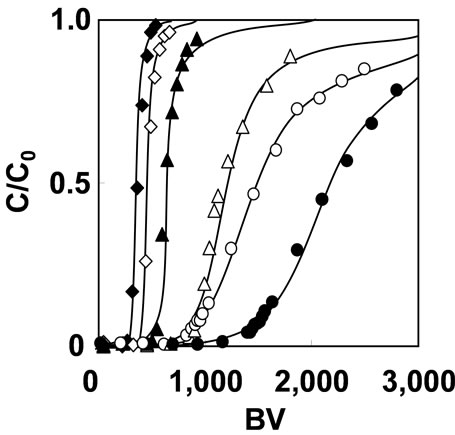
Figure 5. Breakthrough curves of As(V) adsorption with 1 mg/L As(V) solution on SV200 h–1 at various pH. (○) pH 1, (●) pH 2, (△) pH 3, (▲) pH 5, (◇) pH 7 and (◆) pH 9.
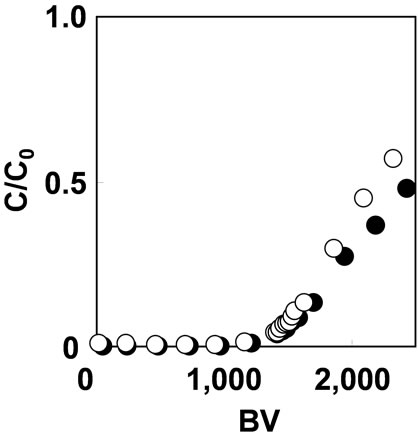
Figure 6. Effect of flow rate on As(V) adsorption with 1 mg/L As(V) solution at pH 2 on (○) SV200 h-1 and (●) SV1000 h–1.
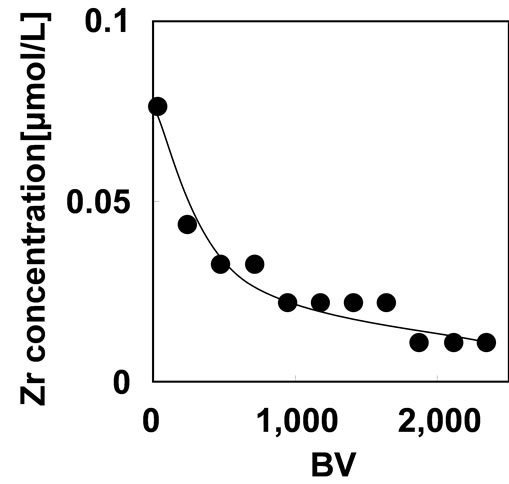
Figure 7. Zirconium(IV) dissolution from the adsorbent during the As(V) adsorption with 1 mg/L of As(V) solution on SV 200 h–1 at pH 2.
3.4. Stability of the Zr-Type Adsorbent
The loaded Zr(IV) should be hold on phosphoric units in stable condition during the adsorption. The evaluation of the stability of the Zr-type adsorbent was carried out by monitoring the Zr(IV) dissolution from the adsorbent during the As(V) adsorption with 1 mg/L of As(V) solution on SV 200 h–1 at pH 2. The result was shown in Figure 7. The concentration of Zr(IV) was 0.08 μmol/L at BV of 20 and then gradually decreased to 0.01 μmol/L.
The total amount of Zr(IV) dissolution from the adsorbent was 0.014 μmol, which correspond to 0.05% of the loaded Zr(IV) on the adsorbent. This result indicates that Zr(IV) is retained on the adsorbent stably.
4. Conclusion
The zirconium(Zr)-type adsorbent using a nonwoven cotton fabric as a trunk polymer was successfully prepared by RIGP with low concentration of phosphoric monomer solution (5%) at low irradiation dose (20 kGy) for short reaction time (2 hours) and was subsequently modified with Zr(IV) by loading on phosphoric units. The adsorption capacity of the adsorbent for As(V) was 0.1 mmol/g with SV 1000 h–1 at pH 2. The adsorption rate of the adsorbent was 100 times faster than that of granular resin. The dissolution of Zr(IV) from the adsorbent was negligible on As(V) adsorption.
5. Acknowledgements
This research was supported by Adaptable and Seamless Technology transfer Program of Japan Science and Technology Agency.
REFERENCES
- P. L. Smedley and D. G. Kinniburgh, “A Review of the Source, Behaviour and Distribution of Arsenic in Natural Water,” Applied Geochemistry, Vol. 17, No. 5, 2002, pp. 517-568. Hdoi:10.1016/S0883-2927(02)00018-5
- P. Ravenscroft, “Arsenic Pollution of Groundwater in Bangladesh,” Encyclopedia of Environmental Health, 2011, pp. 181-192. http://www.sciencedirect.com/science/article/pii/B9780444522726003470
- Y. Kikawada, S. Kawai, K. Shimada and T. Oi, “Origin and Fate of Dissolved Arsenic in Acidic Rivers in the Kusatsu Hot Spring Area, Gunma, Japan,” Geochimica et Cosmochimica Acta, Vol. 70, No. 18, 2006, p. 317. Hdoi:10.1016/j.gca.2006.06.640
- B. K. Mandal and K. T. Suzuki, “Arsenic round the World: A Review,” Talanta, Vol. 58, No. 1, 2002, pp. 201-235. Hdoi:10.1016/S0039-9140(02)00268-0
- C. Hu, H. Liu, G. Chen and J. Qu, “Effect of Aluminum Speciation on Arsenic Removal during Coagulation Process,” Separation and Purification Technology, Vol. 86, No. 2012, pp. 35-40. Hdoi:10.1016/j.seppur.2011.10.017
- C. Lenoble, C. Chabroullet, R. Shukry, B. Serpaud, V. Deluchat and J. C. Bollinger, “Dynamic Arsenic Removal on a MnO2-Roaded Resin,” Journal of Colloid and Interface Science, Vol. 280, No. 1, 2004, pp. 62-67. Hdoi:10.1016/j.jcis.2004.07.034
- T. M. Suzuki, J. O. Bomani, H. Matsunaga and T. Yokoyama, “Preparation of Porous Resin Loaded with Crystalline Hydrous Zirconium Oxide and Its Application to the Removal of Arsenic,” Reactive & Functional Polymers, Vol. 43, No. 1-2, 2000, pp. 165-172. Hdoi:10.1016/S1381-5148(99)00038-3
- Y. M. Zheng, S. F. Lim and J. P. Chen, “Preparation and Characterization of Zirconium-Based Magnetic Sorbent for Arsenate Removal,” Journal of Colloid and Interface Science, Vol. 338, No. 1, 2009, pp. 22-29. Hdoi:10.1016/j.jcis.2009.06.021
- M. Tamada, N. Seko and F. Yoshii, “Application of Radiation-Graft Material for Metal Adsorbent and Crosslinked Natural Polymer for Healthcare Product,” Radiation Physics and Chemistry, Vol. 71, No. 1-2, 2004, pp. 221-225. Hdoi:10.1016/j.radphyschem.2004.03.044
- K. Saito, S. Yamada, S. Furusaki, T. Sugo and J. Okamoto, “Characteristics of Uranium Adsorption by Amidoxime Membrane Synthesized by Radiation-Induced Graft Polymerization,” Journal of Membrane Science, Vol. 34, No. 3, December 1987, pp. 307-315. Hdoi:10.1016/S0376-7388(00)83171-3
- N. Seko, H. Hoshina, N. Kasai, Y. Ueki, M. Tamada, T. Kiryu, K. Tanaka and M. Takahashi, “Novel System for Recovering Scandium from Hot Spring Water with Fibrous Graft Adsorbent,” Journal of Ion Exchange, Vol. 21, No. 3, 2010, pp. 117-122.
- H. Hoshina, N. Seko, Y. Ueki and M. Tamada, “Synthesis of Graft Adsorbent with N-Methyl-D-glucamine for Boron Adsorption,” Journal of Ion Exchange, Vol. 18, No. 4, 2007, pp. 236-239. Hdoi:10.5182/jaie.18.236
- H. Ma, K. Morita, H. Hoshina and N. Seko, “Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether,” Materials Sciences and Applications, Vol. 2, No. 7, 2011, pp. 776-784. Hdoi:10.4236/msa.2011.27107
- F. Basuki, N. Seko, M. Tamada, T. Sugo and T. Kume, “Direct Synthesis of Adsorbent Having Phosphoric Acid with Radiation Induced Graft-Polymerization,” Journal of Ion Exchange, Vol. 14, 2003, pp. 209-212. doi:10.5182/jaie.14.Supplement_209H
NOTES
*Corresponding author.

