International Journal of Organic Chemistry
Vol. 2 No. 3A (2012) , Article ID: 23342 , 4 pages DOI:10.4236/ijoc.2012.223039
![]() /SnO2-Catalyzed C3-Alkylation of 4-Hydroxycoumarin with Secondary Benzyl Alcohols and O-Alkylation with O-Acetyl Compounds
/SnO2-Catalyzed C3-Alkylation of 4-Hydroxycoumarin with Secondary Benzyl Alcohols and O-Alkylation with O-Acetyl Compounds
1School of Chemical Sciences, Swami Ramanand Teerth Marathwada University, Nanded, India
2Department of Chemistry, AP-IIIT Basar, Rajiv Gandhi University of Knowledge Technologies, Hyderabad, India
Email: *ravivarala@gmail.com
Received July 26, 2012; revised August 28, 2012; accepted September 3, 2012
Keywords: C-C and C-O Bond Formations; Sulfated Tin Oxide (STO); Reusability; 4-Hydroxy Coumarin; Secondary Benzyl Alcohol; Secondary Benzyl O-Acetate
ABSTRACT
Sulfated tin oxide (STO) has been found to be an efficient reusable solid superacid catalyst for C3-alkylation and O-alkylation of 4-hydroxycoumarins with benzylic, allylic alcohols/and corresponding acetates respectively, in acetic acid under reflux conditions with good yield of products.
1. Introduction
Coumarin is a privileged scaffold among heterocyclic and are known to possess a wide range of biological activities including antibiotic, anti-malarial, antifungal, anti-viral, and cytotoxic [1-8]. In particular, the 4-hydroxycoumarins and its derivatives (3-alkylated) have evoked a great deal of interest due to their utility as “anticoagulant rodenticides as well as antithrombotic agents” such as warfarin, brodifacoum, difethialone, bromadiolone, coumatetralone, and flocoumafen [9] (Figure 1) and also as nonpeptide human immunodeficiency virus (HIV) protease inhibitors [10]
The C3 or O-alkylation of 4-hydroxycoumarin (formation of new C-C and C-O bond) is undoubtedly one of the most important and challenging reactions in synthetic chemistry due to its pharmaceutical utility as mentioned above and also can be diversified to synthesize 3,4-substitued compounds [11-14].
Although there are several reports about the C3-alkylation of 4-hydroxycoumarins, most of them need organic halides or boronic acid as substrates by Pd-catalyzed C-C bond formation or base mediated alkylation reactions [15-19]. From the synthetic point of view, alcohols are an attractive source compared to the corresponding halides or boronic acid because of easy availability of starting materials and the generation of water as the only side product.
Alternatively, the alkylation can also be performed under acidic conditions with alcohols as alkylating agents, which is not well explored. A few methods that have been reported for the C3-alkylation of 4-hydroxycoumarin so far with alcohols including strong acids, such as HCl, H2SO4, etc. [20-22], Yb(OTf)3 [23], FeCl3.6H2O [24], Amberlite IR-120 [25], molecular iodine [26], Bi(OTf)3 [27], Fe(ClO4)3.xH2O [28], TMSOTf [29], Bi(NO3)3.5H2O/Ionic liquid system [30], Ir-Sn bimetallic system [31]. However, processes involving conventional acids, are inherently associated with problems such as high toxicity, corrosion, catalyst waste and difficulty in separation and recovery. Some of these catalytic systems have several limitations such as longer reaction times, lack of reusability, poor yields, as well.
Therefore, the development of a new efficient, catalytic method for the direct C3-alkylation of hydroxylcoumarin using alcohols is of greater importance and highly desirable.
In recent years, non-polluting and efficient catalytic technologies are much required, considering that environmental restrictions on emissions are covered in several legislations throughout the world. The substitution of homogeneous liquid acids by heterogeneous solid superacids as catalysts is expected to ease their separation from reaction mixture, less corrosion, allowing continuous operation as well as regeneration and neutralization of the catalyst and lowering the cost of process installation and maintenance [32,33]. Sulfated metal oxides with

Figure 1. Prominet 3-(benzyl)-substituted 4-hydroxy coumarins.
both Brønsted and Lewis acid sites are widely used as solid super acid catalysts [34,35]. Sulfated metal oxides are stable to moisture, air and heat [36]. They are easy to prepare and environmentally benign [37].
However, in past few years, considerable attention has been given to sulphated tin oxide (hereafter, STO) which is known to possess strongest surface acidic sites [38-41]. STO was found to be efficient and suitable in Mukaiyama aldol condensation reactions, particularly for benzoylation, transesterfication of keto esters, various gas phase reactions, such as hydration, dehydration, alkylation, isomerization, esterification and polymerization reactions, biodiesel production, β-acetamido ketones and aryl dibenzo [a.j]xanthenes, 2,4,5-Triaryl-1H-imidazole, 2,4- diphenyl-4,6,7, 8-tetrahydro chromen-5-one [42-50].
The catalyst can be repeatedly used without sacrificing its catalytic activity, thus rendering heterogeneous character. Moreover, to our knowledge, STO has not yet been explored as a catalyst for the C3- and O-alkylation of 4- hydroxycoumarins.
In continuation of our interest in developing novel synthetic methodologies, particularly carbon-carbon, carbonheteroatom bond formations [51-57], here in we report our brief findings for a highly efficient method for the C-C bond and C-O bond formation via STO catalyzed C3-alkylation and O-alkylation reactions of 4-hydroxycoumarins with 20 benzylic alchols and benzyl O-acetates, respectively.
2. Result and Discussion
Initially, the reaction of 4-hydroxycoumarin (1, 1 mmol) and 4-methoxy-1-phenylethanol (2b, 1.1 mmol) in the presence of STO was chosen as model reaction to develop optimum reaction conditions (Table 1).
It is evident that the reaction does not progress at RT/reflux (Table 1, entry 9) or in the absence of catalyst. Next, different solvents were screened. We found that a remarkable solvent effect, as acetic acid proved to be the suitable solvent for obtaining good yields under reflux conditions for 5 h (Table 1, entry 6). Other solvents such as MeOH, EtOH, CH3CN, IPA gave no product or considerably decreased yields of the products (Table 1, entries 1-4). Toluene gave moderate yield, although it took longer reaction time.
A catalytic amount of STO (10 mol%) was sufficient to afford the desired product in good yield. No significant improvements in yields were observed on increasing the catalyst loading (Table 1, entry 8). When the reaction was catalyzed by 5 mol% STO, the reaction time was prolonged to 20 h and the desired product (3b) was obtained with only 60% (Table 1, entry 7). The catalyst could be reused for at least three cycles after activation at 400˚C - 500˚C for 1 h; there was a slight decrease in activity after the third use in the model reaction forming 3b (72%). Thus, the most suitable reaction conditions for the formation of 3b were established (Table 1, entry 6).
With the optimized reaction conditions in hand (10 mol% STO, AcOH, reflux), we then evaluated the scope of the benzylation of 4-hydroxycoumarin 1 using a variety of structurally divergent reactants and the results are summarized in Table 2.
We obtained the corresponding C3-alkylated products in 68% - 81% yields, after 5 h (entries 1 - 3, Table 2). The reaction gave higher yields when benzylic alcohols bear electron-donating groups such as methoxy (entry 2, Table 2). When nitro group containing benzylic alcohol was introduced instead of bromoor methoxy, the reaction did not proceed at all. Intrigued by these results, we adapted this protocol to synthesize an anti-coagulant compound, Coumatetralyl (C, Scheme 1; 3d, Table 2) in 70% yield using 4-hydroxycoumarin with 1, 2, 3, 4-tet-

Table 1. Screening for the reaction conditionsa.

Table 2. C3-alkylation of 4-hydroxycomarin with various alcohols.
rahydro-1-napthanol (2d). Moreover, we performed this reaction with substituted allylic alcohols (entries 5 - 7 and 9, Table 2) and excellent yields were obtained. When primary benzyl alcohols were used, they failed to give the expected product. These results clearly demonstrate that the direct C3-alkylation of 4-hydroxycoumarin was successful only with secondary benzylic alcohols. Reaction of benzhydrol (2 h) with 4-hydroxycoumarin did not proceed even after refluxing for 20 h (entry 8, Table 2).
After successful C3-alkylation of 4-hydroxycoumarin with secondary benzyl alcohols (new C-C bond formation), we turned our attention to test the feasibility of secondary benzyl acetates (prepared instantly for the purpose) reacting with 4-hydroxycoumarin under the above optimized conditions to generate novel compounds (5a-e) moderate to good yields (67% - 82%) in the specified time (Table 3). This strategy is successfully applied to new C-O bond formation reaction using STO catalysis.
Unexpectedly, when prenyl acetate (4f) was reacted with 4-hydroxycoumarin, we isolated pyranocoumarin in

Table 3. O-allkylation of 4-hydroxycomarin (1) with various acetates.
good yield (entry 6, Table 3) instead of expected O-alkylated product. This method provides a mild and straightforward route to multi-substituted pyranocoumarins [58-60].
The proposed mechanism of this reaction may tentatively be visualized to occur via a tandem sequence of reactions as depicted in Figure 2 involving removal of water molecule as by-product via formation of stabilized carbocation to act as the alkylating species, derived from alcohol (or else by formation of dimeric ether) in presence of STO using its Bronsted acidic site (C3 –Alkylation). Enolic hydroxyl group is activated by Sn metal via Lewis acid catalysis to make the 3-position more nucleophilic. Whereas, in case of O-alkylation, activation of carbonyl functionality of acetate making it as leaving group by Sn via Lewis acid catalysis and then the formed stabilized carbocation reacts with enolic hydroxide leaving AcOH as byproduct.
3. Conclusions
In summary, in this preliminary communication, we have successfully employed STO as an efficient catalyst to promote C3-benzylation of 4-hydroxycoumarin using secondary benzyl alcohols such as benzylic and allylic alcohols, and also O-alkylation using secondary benzyl acetates. The advantages of this protocol are broad scope, mild conditions, use of inexpensive reusable catalyst, and simplicity of operation since water and acetic acid (solvent) are the only side products, respectively. This method also provides a mild and straightforward route to multi-substituted pyranocoumarins.
Further exploration of the full applicability for the STO catalyzed C3-alkylation and O-alkylation using structurally and electronically divergent substrates with substituted 4-hydroxy coumarins, their scope, limitations and biological activity studies is currently underway.
4. Experimental
STO was prepared according to the literature report [45]. All melting points were determined on an Electrothermal Gallenkamp apparatus. 1H and 13C NMR spectra were recorded on a Varian Gemini Spectrometer 300 MHz. IR spectra were recorded on Nicolet Fourier Transform spectrometer. Mass spectra were obtained on a 7070H or VG Autospec Mass spectrometer using LSIMS technique. Thin-layer chromatography (TLC) was performed on GF-25U (Anal. Tech) plates and silica gel glass-backed plates. Routine column chromatography was conducted using silica gel 100 - 200 mesh.
General experimental procedure for the C3-alkylation of 4-hydroxycoumarins: to a mixture of 4- hydroxycoumarin (1, 1.0 mmol) and secondary benzyl alcohol (2a-h, 1.1 mmol) in acetic acid (10 mL), STO (0.1 mmol) was added and the reaction mixture was stirred for the given time (see Table 2) at reflux tempera-
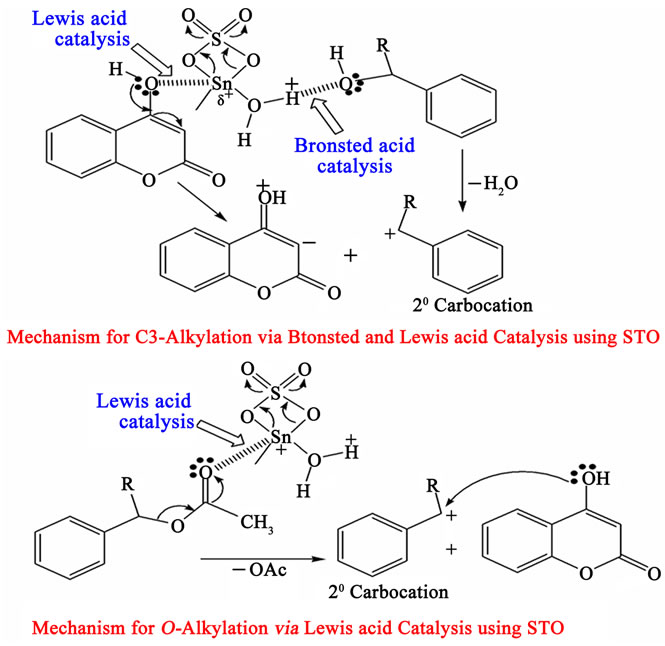
Figure 2. Mechanism for C and O-Alkylation using STO.
ture. After completion of the reaction (monitored by TLC), the reaction mixture was added into water. Adjusted to pH neutral with sodium carbonate and extracted in ethyl acetate. The organic phase was dried over anhydrous Na2SO4 and evaporated under vacuum. The residue was purified by silica gel column with petroleum ether/ethyl acetate (1:3) as eluent to afford the corresponding C3-alkylated 4-hydroxycoumarin (3a-g).
General experimental procedure for the O-alkylation of 4-hydroxycoumarins: To a mixture of 4-hydroxycoumarin (1, 1.0mmol) and secondary O-acetyl compound (4a-f, 1.1 mmol) in acetic acid (10 mL), STO (0.1 mmol) was added and the reaction mixture was stirred for the given time (see Table 3) at reflux temperature. After completion of the reaction (monitored by TLC), to the reaction mixture was added into water. Adjust pH neutral with sodium carbonate and extracted in ethyl acetate. The organic phase was dried over anhydrous Na2SO4 and evaporated under vacuum. The residue was purified by silica gel column with petroleum ether/ethyl acetate (1:3) as eluent to afford the corresponding O-alkylated 4-hydroxycoumarin (5a-f).
Spectral data for known compounds: 3a,27 3b,26 3c,27 3d,31 3e,30 3g26.
Characterization data for new compounds: 3-((E)- 3-(4-chlorophenyl)-1-phenylallyl)-4-hydroxy-2H-chromen-2-one (3f): Pale yellow solid, mp: 168˚C - 171˚C. IR (KBr): ν 3327, 1674, 1626, 1611, 1494, 1393, 1200, 756 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.81 - 7.72 (m, 2H), 7.57 - 7.28 (m, 11H), 6.78 - 6.68 (m, 1H), 6.48 (d, J = 16.4 Hz, 1H), 5.46 (d, J = 6 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 162.9, 160.9, 152.5, 140.1, 136.2, 133.7, 132.2, 1130.0, 128.7, 128.2, 127.9, 127.6, 126.6, 124.1, 123.2, 116.5, 115.8, 106.5, 44.0 ppm. MS (ESI): m/z (rel. abund.%) 389 (M+, 100), 391 (M+, 30) ([M+1]+).
4-(1-Phenylethoxy)-2H-chromen-2-one (5a). Off white solid; mp: 214˚C - 218˚C. IR (KBr): ν 1669, 1621, 1492, 1401, 1218, 1168, 741 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.64 (d, J = 12.4 Hz, 1H), 7.64 - 7.43 (m, 5H), 7.64 - 7.42 (m, 2H), 7.23 (dd, J = 10.8 Hz, 1H), 5.99 (br s, 1H), 4.74 (q, J = 9.6 Hz, 1H), 1.68 (d, J = 9.6 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 163.8, 160.0, 152.6, 141.8, 132.1, 129.8, 127.7, 127.5, 123.9, 123.0, 116.3, 116.2, 110.3, 34.8, 16.8 ppm. MS (ESI): m/z (rel. abund.%) 267.3 ([M+1]+, 100).
4-(1-(4-methoxyphenyl) ethoxy)-2H-chromen-2-one (5b): Off white solid. mp: 180˚C - 184˚C. IR (KBr): 1673, 1628, 1514, 1249 cm–1. 1H NMR (300 MHz, CDCl3): δ 7.70 (dd, J = 8.8Hz, 1H), 7.48 - 7.52 (m, 2H), 7.41 (d, J = 11.2 Hz, 1H), 7.26 - 7.22 (m, 2H), 6.99 (d, J = 4 Hz, 2H), 6.04 (s, 1H), 4.65 (q, J = 10 Hz, 1H), 3.79 (s, 3H), 1.60 (d, J = 9.6 Hz, 3H). ppm. 13C NMR (100 MHz, CDCl3): δ 163.6, 159.8, 159.1, 152.3, 133.1, 131.7, 128.5, 123.8, 122.6, 116.2, 116.1, 114.9, 110.0, 55.3, 33.6, 16.8 ppm. MS (ESI): m/z (rel. abund.%) 297.2 ([M+1]+ , 100).
4-((E)-1,3-diphenylallyloxy)-2H-chromen-2-one (5c): Pale yellow solid, mp: 132˚C - 136˚C. IR (KBr): ν 1678, 1626, 1613, 1501, 1394, 1203, 757 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.78 (d, J = 7.2 Hz, 1H), 7.55 (t, J = 8 Hz, 1H), 7.24 - 7.64 (m, 12H), 6.94 (br, s 1H), 6.67 (dd, J = 6.4, 9.6 Hz, 1H), 6.52 (d, J = 16.4 Hz, 1H), 5.47 (d, J = 5.6 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 163.3, 161.5, 152.4, 139.7, 136.3, 133.9, 132.4, 129.2, 128.7, 128.2, 128.7, 127.7, 126.4, 124.4, 123.1, 116.5, 115.7, 106.4, 43.5 ppm. MS (ESI): m/z (rel. abund.%) 355.0 ([M+1]+ ,100).
4-((E)-3-(4-chlorophenyl)-1-phenylallyloxy)-2H-chromen-2-one (5d): Pale yellow solid, mp: 154˚C - 158˚C. IR (KBr): 1676, 1629, 1616, 1502, 1398, 1210, 758 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.75 (dd, J = 8, 14.8 Hz, 1H), 7.41 - 7.14 (m, 12H), 6.78 - 6.67 (m, 1H), 6.48 (d, J = 16.4 Hz, 1H), 6.41 - 6.35 (m, 1H), 5.44 (dd, J = 5.9 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 163.4, 161.1, 152.8, 139.7, 136.2, 133.7, 132.4, 129.6, 128.4, 128.1, 128.0, 127.8, 126.5, 124.1, 123.3, 116.7, 115.6, 106.5, 43.9 ppm. MS (ESI): m/z (rel. abund.%) 387 (M−,100), 389 (M−, 30) ([M-1]−).
4-(1,2,3,4-tetrahydronaphthalen-4-yloxy)-2H-chromen-2-one (5e): Off white solid, mp: 178˚C - 180˚C. IR (KBr): 2938, 1674, 1628, 1389, 1214, 1148, 751 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.65 (dd, J = 12.8 Hz, 1H), 7.52 (t, 1H), 7.34 - 7.21 (m, 6H), 5.78 (s, 1H), 4.60 (t, J = 10 Hz, 1H), 2.93 (t, J = 8.8 Hz, 2H), 2.25 - 2.20 (m, 1H), 1.94-1.80 (m, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 164.0, 160.3, 152.6, 138.1, 134.7, 132.0, 130.7, 129.4, 128.3, 127.8, 124.1, 123.3, 116.4, 116.1, 109.4, 36.5, 30.3, 29.8, 22.1 ppm. MS (ESI): m/z (rel. abund.%) 293 ([M+1]+ ,100).
3,4-dihydro-2,2-dimethylpyrano[3,2-c]chromen-5(2H)-one (5f): Semi solid. IR (KBr): 1721, 1636, 1614, 1497, 1451, 1383, 1276, 1203, 1171, 1118, 1016, 764, 698 cm−1. 1H NMR (300 MHz, CDCl3): δ 8.18 (dd, J = 10 Hz, 1H), 7.61 - 7.57 (m, 1H), 7.39 - 7.30 (m, 2H), 2.66 (t, J = 6.8 Hz, 2H), 1.87 (t, J = 6.4 Hz, 2H) 1.47 (s, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 162.03, 60.1, 150.3, 128.4, 125.5, 121.6, 117.4, 100, 78.2, 35.5, 27.7, 15.8 ppm. MS (ESI): m/z (rel. abund.%) 231.3 ([M+1]+ ,100).
5. Acknowledgements
V. R. Narayana thanks Dr. Rajanna, OU for constant support and help. Dr. Ravi Varala thanks Prof. Rajendra Sahu (Director, IIIT Basar) and Prof. R. V. Raja Kumar (Vice Chancellor, RGUKT) for their encouragement.
REFERENCES
- R. D. H. Murray, J. Mendez and S. A. Brown, “The Natural Coumarins: Occurrence, Chemistry, and Biochemistry,” Wiley, New York, 1982.
- B. Naser-Hijazi, B. Stolze and K. S. Zanker, “Second Proceedings of the International Society of Coumarin Investigators,” Springer, Berlin, 1994.
- C. Spino, M. Dodier and S. Sotheeswaran, “Anti-HIV Coumarins from Calophyllum Seed Oil,” Bioorganic & Medicinal Chemistry Letters, Vol. 8, No. 24, 1998, pp. 3475- 3478. doi:10.1016/S0960-894X(98)00628-3
- A. Murakami, G. Gao, M. Omura, M. Yano, C. Ito, H. Furukawa, D. Takahashi, K. Koshimizu and H. Ohigashi, “1,1-Dimethylallylcoumarins Potently Supress both Lipopolysaccharideand Interferon-γ-Induced Nitric Oxide Generation in Mouse Macrophage RAW 264.7 Cells,” Bioorganic & Medicinal Chemistry Letters, Vol. 10, No. 1, 2000, pp. 59-62. doi:10.1016/S0960-894X(99)00578-8
- Y. Xia, Z.-Y. Yang, P. Xia, T. Hackl, E. Hamel, A. Mauger, J.-H. Wu and K.-H. Lee, “Antitumor Agents. 211. Fluorinated 2-Phenyl-4-Quinolone Derivatives as Antimitotic Antitumor Agents,” Journal of Medicinal Chemistry, Vol. 44, No. 23, 2001, pp. 3932-3936. doi:10.1021/jm0101085
- M. Itoigawa, C. Ito, H. T.-W. Tan, M. Kuchide, H. Tokuda, H. Nishino and H. Furukawa, “Cancer Chemopreventive Agents, 4-Phenylcoumarins from Calophyllum inophyllum,” Cancer Letters, Vol. 169, No. 1, 2001, pp. 15-19. doi:10.1016/S0304-3835(01)00521-3
- T. Yamaguchi, T. Fukuda, F. Ishibashi and M. Iwao, “The First Total Synthesis of Lamellarin α 20-Sulfate, a Selective Inhibitor of HIV-1 Integrase,” Tetrahedron Letters, 2006, Vol. 47, No. 22, pp. 3755-3757. doi:10.1016/j.tetlet.2006.03.121
- Y. Yamamoto and M. Kurazono, “A New Class of AntiMRSA and Anti-VRE Agents: Preparation and Antibacterial Activities of Indole-Containing Compounds,” Bioorganic Medicinal Chemistry Letters, Vol. 17, No. 6, 2007, pp. 1626-1628. doi:10.1016/j.bmcl.2006.12.081
- I. Manolov and N. D Danchev, “Synthesis and Pharmacological Investigations of Some 4-Hydroxycoumarin Derivatives,” Archiv der Pharmzie, Vol. 336, No. 2, 2003, pp. 83-94. doi:10.1002/ardp.200390010
- Z. Ivezic and M. Trkovnik, “Products of Condensations of Hydroxycoumarin Derivatives with Aromatic and Aliphatic Dialdehydes, Their Preparation and Antiviral Action Thereof,” Pliva Pharm & Chem Works, No. WO 2003029237, 2003,
- A. Estévez-Braun and A. G. González, “Coumarins,” Natural Product Reports, Vol. 14, No. 5, 1997, pp. 465- 475. doi:10.1039/np9971400465
- A. Clerici and O. Porta, “A Novel Synthesis of 4-Hydroxy-3-Phenylcoumarins by Titanum(III)-Mediated Reductive C-C Bond Formation,” Synthesis, Vol. 1993, No. 1, 1993, pp. 99-102. doi:10.1055/s-1993-25808
- T. Mizuno, I. Nishiguchi, T. Hirashima, A. Ogawa, N. Kambe and N. Sonoda, “Facile Synthesis of 4-Hydroxycoumarins by Sulfur-Assisted Carbonylation of 2’-Hydroxyacetophenones with Carbon Monoxide,” Synthesis, Vol. 1988, No. 3, 1988, pp. 257-258. doi:10.1055/s-1988-27537
- S. Wang, G. W. A. Milne, X. Yan, I. J. Posey, M. C. Nicklaus, L. Graham and W. G. Rice, “Discovery of Novel, Non-Peptide HIV-1 Protease Inhibitors by Pharmacophore Searching,” Journal of Medicinal Chemistry, Vol. 39, No. 10, 1996, pp. 2047-2054. doi:10.1021/jm950874+
- D. U. Chen, P. Y. Kuo and D. Y. Yang, “Design and Synthesis of Novel Diphenacoum-Derived, Conformation-Restricted Vitamin K 2,3-Epoxide Reductase Inhibitors,” Bioorganic Medicinal Chemistry Letters, Vol. 15, No. 10, 2005, pp. 2665-2668. doi:10.1016/j.bmcl.2005.03.005
- J. Kischel, D. Michalic, A. Zapf and M. Beller, “FeCl3- Catalyzed Addition of 1,3-Dicarbonyl Compounds to Aromatic Olefins,” Chemistry-Asian Journal, Vol. 2, No. 7, 2007, pp. 909-914. doi:10.1002/asia.200700055
- ] J. Xie, L. Yue, W. Chen, W. Du, J. Zhu, J. Deng and Y. Chen, “Highly Enantioselective Michael Addition of Cyclic 1,3-Dicarbonyl Compounds to α, β-Unsaturated Ketones,” Organic Letters, Vol. 9, No. 3, 2007, pp. 413-415. doi:10.1021/ol062718a
- M. I. Naumov, S. A. Sutirin, A. S. Shavyrin, O. G. Ganina, I. P. Beletskaya, V. B. Rey, S. Combes, J. P. Finet and A. Y. Fedorov, “Cascade Synthesis of Polyoxygenated 6H,11H-Benzopyrano-[4,3-c]benzopyran-11- ones,” Journal of Organic Chemistry, 2007, Vol. 72, No. 9, pp. 3293-3301. doi:10.1021/jo062592v
- G. Toth, S. Moinar, T. Tamas and I. Borbely, “A Simple Procedure for the Alkylation of 4-Hydroxycoumarins at C-3 Position,” Organic Preparation & Procedures International, 1999, Vol. 31, No. 2, pp. 222-225. doi:10.1080/00304949909355718
- E. Enders, “Kondensation von 4-Oxycumarin mit Diarylund Aryl-Alkyl-Carbinolen,” Angewandte Chemie, Vol. 69, No. 13-14, 1957, p. 481. doi:10.1002/ange.19570691318
- R. Ziegler, “Zur Chemie des 4-Hydroxy-Cumarins VIII. Mitteilung: Eine Synthese blutgerinnunghemmender Stoffe,” Monatshefte für Chemie, Vol. 88, No. 1, 1957, p. 25. doi:10.1007/BF01075426
- V. K. Ahluvalia, K. K. Arora and I. Mukherjee, Indian Journal of Chemistry, Section B, Vol. 24B, 1985, p. 298.
- W. Huang, J. Wang, Q. Shen and X. Zhou, “Yb(OTf)3- Catalyzed Propargylation and Allenylation of 1,3-Dicarbonyl Derivatives with Propargylic Alcohols: One-Pot Synthesis of Multi-Substituted Furocoumarin,” Tetrahedron, Vol. 63, No. 47, 2007, pp. 11636-11643. doi:10.1016/j.tet.2007.08.114
- J. KischelJ, K. Mertins, D. Michalik, A. Zapf and M. Beller, “A General and Efficient Iron-Catalyzed Benzylation of 1,3-Dicarbonyl Compounds,” Advanced Synthesis Catalysis, Vol. 349, No. 6, 2007, pp. 865-870. doi:10.1002/adsc.200600497
- C. R. Reddy, B. Srikanth, R. Narsimha and D. S. Shin, “Solid-Supported Acid-Catalyzed C3-Alkylation of 4- Hydroxycoumarins with Secondary Benzyl Alcohols: Access to 3,4-Disubstituted Coumarins via Pd-Coupling,” Tetrahedron, 2008, Vol. 64, No. 51, pp. 11666-11672. doi:10.1016/j.tet.2008.10.017
- X. Lin, X. Dai, Z. Mao and Y. Wang, “Molecular IodineCatalyzed C3-Alkylation of 4-Hydroxycoumarins with Secondary Benzyl Alcohols,” Tetrahedron, Vol. 65, No. 45, 2009, pp. 9233-9237. doi:10.1016/j.tet.2009.09.007
- R. Rueping, B. J. Nachtsheim and E. Sugiono, “Direct Catalytic Benzylation of Hydroxycoumarin-Efficient Synthesis of Warfarin Derivatives and Analogues,” Synlett, Vol. 2010, No. 10, 2010, pp. 1549-1553. doi:10.1055/s-0029-1219936
- P. Thirupathi and S. S. Kim, “Fe(ClO4)3·xH2O-Catalyzed direct C-C Bond Forming Reactions between Secondary Benzylic Alcohols with Different Types of Nucleophiles,’’ Tetrahedron, Vol. 66, No. 16, 2010, pp. 2995- 3003. doi:10.1016/j.tet.2010.02.063
- P. Theerthagiri and A. Lalitha, “Benzylation of β-Dicerbonyl Compounds and 4-Hydroxycoumarin Using TMSOTf Catalyst: A Simple, Mild, and Efficient Method,” Tetrahedron Letters, Vol. 51, No. 41, 2010, pp. 5454-5458. doi:10.1016/j.tetlet.2010.08.019
- G. Aridoss and K. K. Laali, “Condensation of Propargylic Alcohols with 1,3-Dicarbonyl Compounds and 4-Hydroxycoumarins in Ionic Liquids (ILs),” Tetrahedron Letters, 2011, Vol. 52, No. 51, pp. 6859-6864. doi:10.1016/j.tetlet.2011.10.021
- P. N. Chatterjee and S. Roy, “Alkylation of 1,3-Dicarbonyl Compounds with Benzylic and Propargylic Alcohols Using Ir-Sn Bimetallic Catalyst: Synthesis of Fully Decorated Furans and Pyrroles,” Tetrahedron, 2011, Vol. 67, No. 25, pp. 4569-4577.
- X. Bokhimi, A. Morales, E. Ortiz, T. Lopez, R. Gomez and J. Navarrete, “Sulfate Ions in Titania Polymorphs,” Journal of Sol-Gel Science Technology, Vol. 29, No. 1, 2004, pp. 31-40. doi:10.1023/B:JSST.0000016135.02238.0e
- F. Lonyi, J. Valyon, J. Engelhardt and F. Mizukami, “Characterization and Catalytic Properties of Sulfated ZrO2-TiO2Mixed Oxides,” Journal of Catalysis, 1996, Vol. 160, No. 2, pp. 279-289. doi:10.1006/jcat.1996.0146
- J. Deutsch, H. A. Prescott, D. Müller, E. Kemnitz and H. Lieske, “Acylation of Naphthalenes and Anthracene on Sulfated Zirconia,” Journal of Catalysis, Vol. 231, No. 2, 2005, pp. 269-278. doi:10.1016/j.jcat.2005.01.024
- M. V. Luzgin, K. Thomas, J. Gestel, J. P. Gilson and A. G. Stepanov, “Propane Carbonylation on Sulfated Zirconia Catalyst as Studied by 13C MAS NMR and FTIR Spectroscopy,” Journal of Catalysis, Vol. 223, No. 2, 2004, pp. 290-295.
- H. Ma, J. Xiao and B. Wang, “Environmentally Friendly Efficient Coupling of n-Heptane by Sulfated Tri-Component Metal Oxides in Slurry Bubble Column Reactor,” Journal of Hazardous Materials, Vol. 166, No. 2-3, 2009, pp. 860-865. doi:10.1016/j.jhazmat.2008.11.096
- D. Zhai, Y. Nie, Y. Yue, H. He, W. Hua and Z. Gao, “Esterification and Transesterification on Fe2O3-Doped Sulfated Tin Oxide Catalysts,” Catalysis Communications, Vol. 12, No. 7, 2011, pp. 593-596. doi:10.1016/j.catcom.2010.12.020
- C. P. Nicholas and T. J. Marks, “Sulfated Tin Oxide Nanoparticles as Supports for Molecule-Based Olefin Polymerization Catalysts,” Nano Letters, Vol. 4, No. 8, 2004, pp. 1557-1559. doi:10.1021/nl049255r
- H. Matsuhashi, H. Miyazaki, Y. Kawamura, H. Nakamura and K. Arata, “Preparation of a Solid Superacid of Sulfated Tin Oxide with Acidity Higher Than That of Sulfated Zirconia and Its Applications to Aldol Condensation and Benzoylation,” Chemistry of Materials, Vol. 13, No. 9, 2001, pp. 3038-3042. doi:10.1021/cm0104553
- M. K. Lam, K. T. Lee and A. R. Mohamed, “Application of Sulfated tin oxide in Transesterification of Waste Cooking Oil: An Optimization Study,” Applied Catalysis B: Environmental, Vol. 93, No. 1-2, pp. 134-139.
- H. Matsuhashi, T. Tanaka and K. Arata, “Measurement of Heat of Argon Adsorption for the Evaluation of Relative Acid Strength of Some Sulfated Metal Oxides and HType Zeolites,” Journal of Physical Chemistry B, Vol. 105, No. 40, 2001, pp. 9669-9671. doi:10.1021/jp0118017
- S. R. Sarda, V. A. Puri, A. B. Rode, T. N. Dalawe, W. N. Jadhav and R. P. Pawar, “Sulfated Tin Oxides: A Suitable Reagent for Synthesis of 2,4-Diphenyl-4,6,7,8-tetrahydrochromen-5-one,” Arkivoc, Vol. 2007, No. xvi, 2007, pp. 246-251.
- M. A. Naik, B. G. Mishra, A. Dubey and G. D. Hota, “Catalytic Applications of Sulfated Tin Oxide for Synthesis of Structurally Diverse β-Acetamido Ketones and Aryl-14H-Dibenzo[a.j]Xanthenes,” Bulletin of Catalysis Society of India, Vol. 8, No. 1, 2009, pp. 35-40.
- M. Sowmiya, A. Sharma, A. Parsodkar, B. G. Mishra and A. Dubey, “Nanosized Sulfated SnO2 Dispersed in the Micropores of Al-Pillared Clay As an Efficient Catalyst for the Synthesis of Some Biologically Important Molecules,’’ Applied Catalysis A, Vol. 333, No. 2, 2007, pp. 272-280. doi:10.1016/j.apcata.2007.09.024
- S. P. Chavan, P. K. Zubaidha, S. W. Dantale, A. Kesavaraja, A. V. Ramaswamy and T. Ravindranathan, “Use of Solid Superacid (Sulphated SnO2) as Efficient Catalyst in Facile Transesterification of Ketoesters,” Tetrahedron, Vol. 37, No. 2, 1996, pp. 233-236. doi:10.1016/0040-4039(95)02136-1
- Q. Lu, W.-M. Xiong, W. Z. Li, Q. X. Guo and X. F. Zhu, “Catalytic Pyrolysis of Cellulose with Sulfated Metal Oxides: A Promising Method for Obtaining High Yield of Light Furan Compounds,” Bioresource Technology, Vol. 100, No. 20, 2009, pp. 4871-4876. doi:10.1016/j.biortech.2009.04.068
- Y. Du, S. S. Liu, Y. Zhang, C. Yin, Y. Di and F.-S. Xiao, “Mesostructured Sulfated Tin Oxide and its High Catalytic Activity in Esterification and Friedel-Crafts Acylation,” Catalysis Letters, Vol. 108, No. 3-4, 2006, pp. 155-158. doi:10.1007/s10562-006-0037-7
- S. Furuta, H. Matsuhashi and K. Arata, “Catalytic Action of Sulfated Tin Oxide for Etherification and Esterification in Comparison with Sulfated Zirconia,” Applied Catalysis A: General, Vol. 269, No. 1-2, 2004, pp. 187-191. doi:10.1016/j.apcata.2004.04.017
- J. I. Moreno, R. James, R. Gómez and M. E. NiñoGómez, “Evaluation of Sulfated Tin Oxides in the Esterification Reaction of Free Fatty Acids,” Catalysis Today, Vol. 172, No. 1, 2011, pp. 34-40. doi:10.1016/j.cattod.2011.03.052
- S. A. Dake, M. B. Khedkar, G. S. Irmale, S. J. Ukalgaonkar, V. V. Thorat, S. A. Shintre and R. P. Pawar, “Sulfated Tin Oxide: A Reusable and Highly Efficient Heterogeneous Catalyst for the Synthesis of 2,4,5-Triaryl-1H-imidazole Derivatives,” Synthetic Communications, Vol. 42, No. 10, 2012, pp. 1509-1520. doi:10.1080/00397911.2010.541744
- V. B. C. Figueira, A. G. Esqué, R. Varala, C. GonzálezBello, S. Prabhakar and A. M. Lobo, “Functional Desymmetrization of 1,3-Dioximes for the Obtention of 1,2,3- Hetero Trisubstituted Carbocycles,” Tetrahedron Letters, Vol. 51, No. 15, 2010, pp. 2029-2031. doi:10.1016/j.tetlet.2010.02.048
- R. Varala, E. Ramu and S. R. Adapa, “Ruthenium (III) Chloride-Catalyzed Efficient Protocol for Ethyl Diazoacetate Insertion into the N-H Bond of Secondary Amines,” Monatshefte für Chemie, Vol. 139, No. 11, 2008, pp. 1369- 1372. doi:10.1007/s00706-008-0927-z
- R. Enugala, S. Nuvvula, V. Kotra, R. Varala and S. R. Adapa, “Green Approach for the Efficient Synthesis of Quinolines Promoted by Citric Acid,” Heterocycles, Vol. 75, No. 10, 2008, pp. 2523-2533. doi:10.3987/COM-08-11405
- E. Ramu, R. Varala, N. Sreelatha and S. R. Adapa, “ZnZn(OAc)2·2H2O: A Versatile Catalyst for the One-Pot Synthesis of Propargylamines,” Tetrahedron Letters, Vol. 48, No. 40, 2007, pp. 7184-7190. doi:10.1016/j.tetlet.2007.07.196
- R. Varala, A. Nasreen, E. Ramu and S. R. Adapa, “LProline Catalyzed Selective Synthesis of 2-Aryl-1-Arylmethyl-1H-Benzimidazoles,” Tetrahedron Letters, Vol. 48, No. 40, 2007, pp. 6972-6976. doi:10.1016/j.tetlet.2006.11.010
- R. Varala, E. Ramu and S. R. Adapa, “Efficient and Rapid Friedlander Synthesis of Functionalized Quinolines Catalyzed by Neodymium (III) Nitrate Hexahydrate,” Synthesis, Vol. 2006, No. 22, 2006, pp. 3825-3830.
- R. Varala, E. Ramu, N. Sreelatha and S. R. Adapa, “Ceric Ammonium Nitrate (CAN) Promoted Efficient Synthesis of 1,5-Benzodiazepine Derivatives,” Synlett, Vol. 2006, No. 7, 2006, pp.1009-1014.
- J. Reisch, “Die Darstellung von Antikoagulantien vom Typ des Warfarin® durch Kondensation von Alkinolen mit 4-Hydroxycumarin,” Archiv der Pharmazie, Vol. 299, No. 9, 1966, pp. 806-808. doi:10.1002/ardp.19662990913
- Y. Acquot, B. Refouvelet, L. Bermont, G. L. Adessi, G. Leclercq and A. Xicluna, “Synthesis and Cytotoxic Activity of New 2,4-Diaryl-4H,5H-pyrano[3,2-c]benzopyran-5-ones on MCF-7 Cells,”Pharmazie, Vol. 57, No. 4, 2002, p. 233.
- [61] G. Appendino, G. Cravotto, L. Toma, R. Annunziata and G. Palmisano, “The Chemistry of Coumarin Derivatives. Part VI. Diels-Alder Trapping of 3-Methylene-2,4-chromandione. A New Entry to Substituted Pyrano[3,2-c] coumarins,” Journal of Organic Chemistry, Vol. 59, No. 19, 1994, pp. 5556- 5564.
NOTES
*Corresponding author.

